
Concept explainers
(a)
Interpretation:
The acceptable name for the following
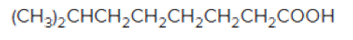
Concept introduction:
An organic compound in which carboxy
In order to give the name to the carboxylic acid group, the following steps are followed:
1. The parent (longest)
2. The ending of the parent chain from alkane (-e) is changed to -oic acid for a carboxylic acid group.
3. The numbering is of the chain is done in such a way that carbonyl carbon gets the smaller number.
4. Name should be written in alphabetical order and other substituents are shown by the number.
For number of carbons atoms chain, the prefix is given as:
Carbon-1 meth
Carbon-2 eth
Carbon-3 prop
Carbon-4 but
Carbon-5 pent
Carbon-6 hex
Carbon-7 hept
Carbon-8 oct
Carbon-9 non
Carbon-10 dec
(b)
Interpretation:
The acceptable name for the following carboxylic acid should be determined:
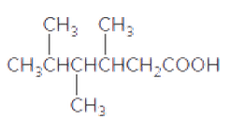
Concept introduction:
An organic compound in which carboxy functional group that is -COOH is bonded to the carbon atom is said to be a carboxylic acid. The general formula for carboxylic acid is RCOOH or RCO2H.
In order to give the name to the carboxylic acid group, the following steps are followed:
1. The parent (longest) alkane chain is identified.
2. The ending of the parent chain from alkane (-e) is changed to -oic acid for a carboxylic acid group.
3. The numbering is of the chain is done in such a way that carbonyl carbon gets the smaller number.
4. Name should be written in alphabetical order and other substituents are shown by the number.
For number of carbons atoms chain, the prefix is given as:
Carbon-1 meth
Carbon-2 eth
Carbon-3 prop
Carbon-4 but
Carbon-5 pent
Carbon-6 hex
Carbon-7 hept
Carbon-8 oct
Carbon-9 non
Carbon-10 dec
(c)
Interpretation:
The acceptable name for the following carboxylic acid should be determined:
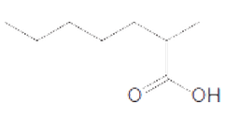
Concept introduction:
An organic compound in which carboxy functional group that is -COOH is bonded to the carbon atom is said to be a carboxylic acid. The general formula for carboxylic acid is RCOOH or RCO2H.
In order to give the name to the carboxylic acid group, the following steps are followed:
1. The parent (longest) alkane chain is identified.
2. The ending of the parent chain from alkane (-e) is changed to -oic acid for a carboxylic acid group.
3. The numbering is of the chain is done in such a way that carbonyl carbon gets the smaller number.
4. Name should be written in alphabetical order and other substituents are shown by the number.
For number of carbons atoms chain, the prefix is given as:
Carbon-1 meth
Carbon-2 eth
Carbon-3 prop
Carbon-4 but
Carbon-5 pent
Carbon-6 hex
Carbon-7 hept
Carbon-8 oct
Carbon-9 non
Carbon-10 dec
Want to see the full answer?
Check out a sample textbook solution
Chapter 17 Solutions
CONNECT IA GENERAL ORGANIC&BIO CHEMISTRY
- true or false The equilibrium constant for this reaction is 0.20. N2O4(g) ⇔ 2NO2(g) Based on the above, the equilibrium constant for the following reaction is 5. 4NO2(g) ⇔ 2N2O4(g)arrow_forwardtrue or false The equilibrium constant for this reaction is 0.20. N2O4(g) ⇔ 2NO2(g) Based on the above, the equilibrium constant for the following reaction is 0.4. 2N2O4(g) ⇔ 4NO2(g)arrow_forwardtrue or false Using the following equilibrium, if heat is added the equilibrium will shift toward the reactants. N2(g) + 3H2(g) ⇔ 2NH3(g) + heatarrow_forward
- True or False Using the following equilibrium, if heat is added the equilibrium will shift toward the products. N2O4(g) + heat ⇔ 2NO2(g)arrow_forwardtrue or false Using the following equilibrium, if solid carbon is added the equilibrium will shift toward the products. C(s) + CO2(g) ⇔ 2CO(g)arrow_forwardProvide the complete mechanism for the reaction below. You must include appropriate arrows,intermediates, and formal charges. Please also provide a reason to explain why the 1,4-adduct is preferred over the 1,3-adduct.arrow_forward
- Which of the following pairs are resonance structures of one another? I. III. || III IV + II. :0: n P !༠ IV. EN: Narrow_forwardPredict the major organic product(s) and byproducts (either organic or inorganic) for thefollowing reactions.arrow_forwardA 8.25 g sample of aluminum at 55°C released 2500 J of heat. The specific heat of aluminum is 0.900 J/g°C. The density of aluminum is 2.70 g/mL. Calculate the final temperature of the aluminum sample in °C.arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co

