
(a)
Interpretation:
The sign and magnitude of
Concept Introduction :
The intermolecular forces in a solute can be broken down by new interactions from the solution as each solute particle will be surrounded by solvent particles in a solution. This is possible when there is disruption between the solute -solute and solvent-solvent interaction.
(a)
Answer to Problem 26E
The values for
Therefore
Explanation of Solution
The process of formation of solution takes place in 3 main steps
- The solutes separating into individual components require energy making it an endothermic reaction.
- The intermolecular forces in solvent must be such that it can make space for solute which requires energy making it an endothermic reaction.
- To allow the solvent and solute molecules to interact which absorbs energy making it an exothermic reaction.
The formation of solution involves enthalpy changes which is depicted as
| Result | |||||
| Non polar solute, Non polar solvent | Small | Small | Small | Small | Solution will form |
| Non-polar solute, polar solvent | Small | Large | Small | Large, positive | No-solution will form |
| Polar solute, Non polar solvent | Large | Small | Small | Large negative | No solution will form |
| Polar solute-polar solvent | Large | Large | Large, negative | small | Solution will form |
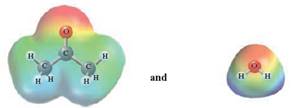
In this reaction, acetone reacts with water and as water is polar in nature, the values of each enthalpy changes are depicted as
| Outcome | |||||
| Polar solute, Non polar solvent | Large | Small | Small | Large negative | No solution will form |
(b)
Interpretation:
The sign and magnitude of
Concept Introduction :
The intermolecular forces in a solute can be broken down by new interactions from the solution as each solute particle will be surrounded by solvent particles in a solution. This is possible when there is disruption between the solute -solute and solvent-solvent interaction.
(b)
Answer to Problem 26E
The values for
Therefore
Explanation of Solution
The process of formation of solution takes place in 3 main steps
- The solutes separating into individual components require energy making it an endothermic reaction.
- The intermolecular forces in solvent must be such that it can make space for solute which requires energy making it an endothermic reaction.
- To allow the solvent and solute molecules to interact which absorbs energy making it an exothermic reaction.
The formation of solution involves enthalpy changes which is depicted as
| Result | |||||
| Non polar solute, Non polar solvent | Small | Small | Small | Small | Solution will form |
| Non-polar solute, polar solvent | Small | Large | Small | Large, positive | No-solution will form |
| Polar solute, Non polar solvent | Large | Small | Small | Large negative | No solution will form |
| Polar solute-polar solvent | Large | Large | Large, negative | small | Solution will form |
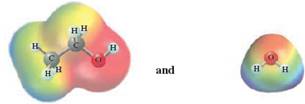
In this reaction, ethanol reacts with water and as water is polar in nature, the values of each enthalpy changes are depicted as
| Outcome | |||||
| Polar solute-polar solvent | Large | Large | Large, negative | small | Solution will form |
(c)
Interpretation:
The sign and magnitude of
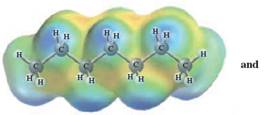
Concept Introduction :
The intermolecular forces in a solute can be broken down by new interactions from the solution as each solute particle will be surrounded by solvent particles in a solution. This is possible when there is disruption between the solute -solute and solvent-solvent interaction.
(c)
Answer to Problem 26E
The values for
Therefore
Explanation of Solution
The process of formation of solution takes place in 3 main steps
- The solutes separating into individual components require energy making it an endothermic reaction.
- The intermolecular forces in solvent must be such that it can make space for solute which requires energy making it an endothermic reaction.
- To allow the solvent and solute molecules to interact which absorbs energy making it an exothermic reaction.
The formation of solution involves enthalpy changes which is depicted as:
| Result | |||||
| Non polar solute, Non polar solvent | Small | Small | Small | Small | Solution will form |
| Non-polar solute, polar solvent | Small | Large | Small | Large, positive | No-solution will form |
| Polar solute, Non polar solvent | Large | Small | Small | Large negative | No solution will form |
| Polar solute-polar solvent | Large | Large | Large, negative | small | Solution will form |
In this reaction, heptane reacts with hexane and as both is non-polar in nature; the values of each enthalpy changes are depicted as:
| Outcome | |||||
| Non polar solute, Non polar solvent | Small | Small | Small | Small | Solution will form |
(d)
Interpretation:
The sign and magnitude of
Concept Introduction :
The intermolecular forces in a solute can be broken down by new interactions from the solution as each solute particle will be surrounded by solvent particles in a solution. This can happen when there is disruption between the solute-solute and solvent-solvent interaction.
(d)
Answer to Problem 26E
The values for
Therefore
Explanation of Solution
The process of formation of solution takes place in 3 main steps
- The solutes separating into individual components require energy making it an endothermic reaction.
- The intermolecular forces in solvent must be such that it can make space for solute which requires energy making it an endothermic reaction.
- To allow the solvent and solute molecules to interact which absorbs energy making it an exothermic reaction.
The formation of solution involves enthalpy changes which are depicted as:
| Result | |||||
| Non polar solute, Non polar solvent | Small | Small | Small | Small | Solution will form |
| Non-polar solute, polar solvent | Small | Large | Small | Large, positive | No-solution will form |
| Polar solute, Non polar solvent | Large | Small | Small | Large negative | No solution will form |
| Polar solute-polar solvent | Large | Large | Large, negative | small | Solution will form |
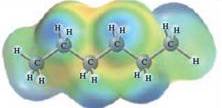
In this reaction, heptane reacts with water wherein heptane is non-polar and water is polar in nature. The values of each enthalpy changes are depicted as:
| Result | |||||
| Non-polar solute, polar solvent | Small | Large | Small | Large, positive | Solution will not form. |
Want to see more full solutions like this?
Chapter 17 Solutions
Chemical Principles
- Determine the structures of the missing organic molecules in the following reaction: X+H₂O H* H+ Y OH OH Note: Molecules that share the same letter have the exact same structure. In the drawing area below, draw the skeletal ("line") structures of the missing organic molecules X and Y. You may draw the structures in any arrangement that you like, so long as they aren't touching. Click and drag to start drawing a structure. X Sarrow_forwardPredict the major products of this organic reaction. If there aren't any products, because nothing will happen, check the box under the drawing area instead. No reaction. HO. O :☐ + G Na O.H Click and drag to start drawing a structure. XS xs H₂Oarrow_forwardWhat are the angles a and b in the actual molecule of which this is a Lewis structure? H H C H- a -H b H Note for advanced students: give the ideal angles, and don't worry about small differences from the ideal groups may have slightly different sizes. a = b = 0 °arrow_forward
- What are the angles a and b in the actual molecule of which this is a Lewis structure? :0: HCOH a Note for advanced students: give the ideal angles, and don't worry about small differences from the ideal that might be caused by the fact that different electron groups may have slightly different sizes. a = 0 b=0° Sarrow_forwardDetermine the structures of the missing organic molecules in the following reaction: + H₂O +H OH O OH +H OH X Note: Molecules that share the same letter have the exact same structure. In the drawing area below, draw the skeletal ("line") structure of the missing organic molecule X. Click and drag to start drawing a structure.arrow_forwardIdentify the missing organic reactant in the following reaction: x + x O OH H* + ☑- X H+ O O Х Note: This chemical equation only focuses on the important organic molecules in the reaction. Additional inorganic or small-molecule reactants or products (like H₂O) are not shown. In the drawing area below, draw the skeletal ("line") structure of the missing organic reactant X. Click and drag to start drawing a structure. Carrow_forward
- CH3O OH OH O hemiacetal O acetal O neither O 0 O hemiacetal acetal neither OH hemiacetal O acetal O neither CH2 O-CH2-CH3 CH3-C-OH O hemiacetal O acetal CH3-CH2-CH2-0-c-O-CH2-CH2-CH3 O neither HO-CH2 ? 000 Ar Barrow_forwardWhat would be the best choices for the missing reagents 1 and 3 in this synthesis? 1. PPh3 2 2. n-BuLi 3 Draw the missing reagents in the drawing area below. You can draw them in any arrangement you like. • Do not draw the missing reagent 2. If you draw 1 correctly, we'll know what it is. • Note: if one of your reagents needs to contain a halogen, use bromine. Explanation Check Click and drag to start drawing a structure.arrow_forwardPredict the products of this organic reaction: NaBH3CN + NH2 ? H+ Click and drag to start drawing a structure. ×arrow_forward
- Predict the organic products that form in the reaction below: + OH +H H+ ➤ ☑ X - Y Note: You may assume you have an excess of either reactant if the reaction requires more than one of those molecules to form the products. In the drawing area below, draw the skeletal ("line") structures of the missing organic products X and Y. You may draw the structures in any arrangement that you like, so long as they aren't touching. Click and drag to start drawing a structure. Garrow_forwardPredict the organic products that form in the reaction below: OH H+ H+ + ☑ Y Note: You may assume you have an excess of either reactant if the reaction requires more than one of those molecules to form the products. In the drawing area below, draw the skeletal ("line") structures of the missing organic products X and Y. You may draw the structures in any arrangement that you like, so long as they aren't touching. Click and drag to start drawing a structure. ✓ marrow_forwardDetermine the structures of the missing organic molecules in the following reaction: + H₂O +H H+ Y Z ☑ ☑ Note: Molecules that share the same letter have the exact same structure. In the drawing area below, draw the skeletal ("line") structures of the missing organic molecules X, Y, and Z. You may draw the structures in any arrangement that you like, so long as they aren't touching. Molecule X shows up in multiple steps, but you only have to draw its structure once. Click and drag to start drawing a structure. AP +arrow_forward
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning





