
Concept explainers
18-26 Answer true or false.
(a)
(b) Phenols, alcohols, and carboxylic acids have in common the presence of an —OH group.
(c) Carboxylic acids are stronger acids than alcohols but weaker acids than phenols.
(d) The order of acidity of the following carboxylic acids is:
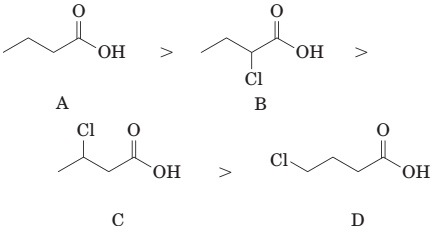
(e) The order of acidity of the following carboxylic acids is:
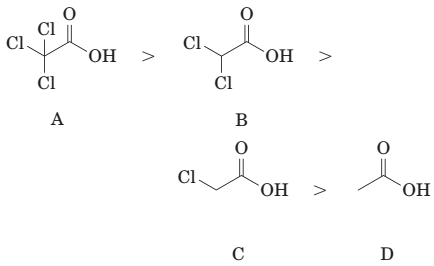
(f) The reaction of benzoic acid with aqueous sodium hydroxide gives sodium benzoate.
(g) A mixture of the following compounds is extracted in order with (1) 1 M HCI, (2) 1 M NaOH, and (3) diethyl ether. Only compound II is extracted into the basic layer.
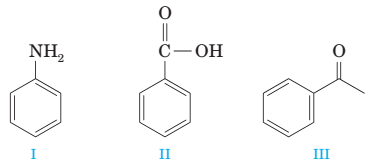
(h) Conversion of compound I to compound II is best accomplished by reduction with NaBH4.
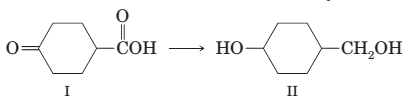
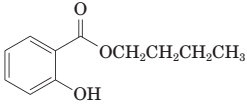
(i) The following ester can be prepared by treating benzoic acid with 1-butanol in the presence of a catalytic amount of H2SO4:
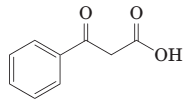
(j) Thermal decarboxylation of this ((-ketoacid gives benzoic acid and carbon dioxide:
(k) Thermal decarboxylation of this (-ketoacid gives 2-pentanone and carbon dioxide.
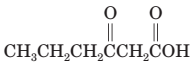
Trending nowThis is a popular solution!

Chapter 17 Solutions
Introduction To General, Organic, And Biochemistry
- 2. Please fill in missing reactants, reagents, reaction conditions, or products in the provided blank boxes OMe ...-CF2-CF2-CF2-CF2-CF2-...arrow_forwardDon't used hand raiting and don't used Ai solutionarrow_forwardI don't understand what to put for final step. Does that just mean termination? And would a radical form when I add bromine to ch2 between the rings?arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co



