
Concept explainers
(a)
Interpretation:
Whether table sugar is a monosaccharide, disaccharide, or polysaccharide is to be stated.
Concept introduction:
On the basis of the structural differences carbohydrates are classified into three parts.
Monosaccharide: They are the polyhydroxy
Disaccharide: They are the polyhydroxy aldehydes or ketones containing two aldehyde or ketone units. For example: Sucrose, lactose.
Polysaccharide: They are the polyhydroxy aldehydes or ketones containing more than two aldehyde or ketone units. For example: Cellulose, starch.
Answer to Problem 17.4E
Table sugar is a disaccharide.
Explanation of Solution
Table sugar is also known as sucrose. Sucrose on hydrolysis gives two monosaccharides, glucose and fructose which are linked through a glycosidic linkage. This shows that sucrose or table sugar is a disaccharide.
Table sugar is a disaccharide.
(b)
Interpretation:
Whether the given carbohydrate is a monosaccharide, disaccharide, or polysaccharide is to be stated.
Concept introduction:
On the basis of the structural differences carbohydrates are classified into three parts.
Monosaccharide: They are the polyhydroxy aldehydes or ketones containing only one aldehyde or ketone unit. For example: glucose and fructose.
Disaccharide: They are the polyhydroxy aldehydes or ketones containing two aldehyde or ketone units. For example: Sucrose, lactose.
Polysaccharide: They are the polyhydroxy aldehydes or ketones containing more than two aldehyde or ketone units. For example: Cellulose, starch.
Answer to Problem 17.4E
The given carbohydrate is an example of monosaccharide.
Explanation of Solution
The structural formula of the given carbohydrate is shown below.
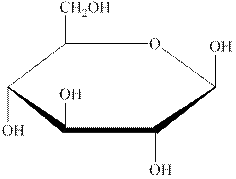
Figure 1
The above structure of carbohydrate shows that there is no glycosidic linkage through which another carbohydrate is linked. Therefore, the above carbohydrate is a monosaccharide.
The carbohydrate shown in Figure 1 is an example of monosaccharide.
(c)
Interpretation:
Whether starch is a monosaccharide, disaccharide, or polysaccharide is to be stated.
Concept introduction:
On the basis of the structural differences carbohydrates are classified into three parts.
Monosaccharide: They are the polyhydroxy aldehydes or ketones containing only one aldehyde or ketone unit. For example: glucose and fructose.
Disaccharide: They are the polyhydroxy aldehydes or ketones containing two aldehyde or ketone units. For example: Sucrose, lactose.
Polysaccharide: They are the polyhydroxy aldehydes or ketones containing more than two aldehyde or ketone units. For example: Cellulose, starch.
Answer to Problem 17.4E
Starch is a polysaccharide.
Explanation of Solution
Starch is the
Starch is an example of polysaccharide.
(d)
Interpretation:
Whether fructose is a monosaccharide, disaccharide, or polysaccharide is to be stated.
Concept introduction:
On the basis of the structural differences carbohydrates are classified into three parts.
Monosaccharide: They are the polyhydroxy aldehydes or ketones containing only one aldehyde or ketone unit. For example: glucose and fructose.
Disaccharide: They are the polyhydroxy aldehydes or ketones containing two aldehyde or ketone units. For example: Sucrose, lactose.
Polysaccharide: They are the polyhydroxy aldehydes or ketones containing more than two aldehyde or ketone units. For example: Cellulose, starch.
Answer to Problem 17.4E
Fructose is a monosaccharide.
Explanation of Solution
Hydrolysis of sucrose produces glucose and fructose which do not undergo further hydrolysis. Therefore, fructose comes under the category of monosaccharide.
Fructose is an example of monosaccharide.
(e)
Interpretation:
Whether cellulose is a monosaccharide, disaccharide, or polysaccharide is to be stated.
Concept introduction:
On the basis of the structural differences carbohydrates are classified into three parts.
Monosaccharide: They are the polyhydroxy aldehydes or ketones containing only one aldehyde or ketone unit. For example: glucose and fructose.
Disaccharide: They are the polyhydroxy aldehydes or ketones containing two aldehyde or ketone units. For example: Sucrose, lactose.
Polysaccharide: They are the polyhydroxy aldehydes or ketones containing more than two aldehyde or ketone units. For example: Cellulose, starch.
Answer to Problem 17.4E
Cellulose is a polysaccharide.
Explanation of Solution
Cellulose is a polymeric form of D-glucose because cellulose on hydrolysis gives a large number of glucose as monosaccharide. Each glucose is linked with
Cellulose on hydrolysis gives a large number of molecules of glucose. As a result, cellulose is a polysaccharide.
(f)
Interpretation:
Whether the given carbohydrate is a monosaccharide, disaccharide, or polysaccharide is to be stated.
Concept introduction:
On the basis of the structural differences carbohydrates are classified into three parts.
Monosaccharide: They are the polyhydroxy aldehydes or ketones containing only one aldehyde or ketone unit. For example: glucose and fructose.
Disaccharide: They are the polyhydroxy aldehydes or ketones containing two aldehyde or ketone units. For example: Sucrose, lactose.
Polysaccharide: They are the polyhydroxy aldehydes or ketones containing more than two aldehyde or ketone units. For example: Cellulose, starch.
Answer to Problem 17.4E
The given carbohydrate is a disaccharide.
Explanation of Solution
The structural formula of the given carbohydrate is shown below.
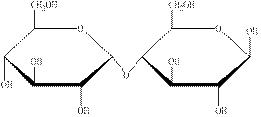
Figure 2
The above structure of carbohydrate shows that two monosaccharides are linked to each other through glycosidic linkage and results in the formation of a disaccharide. Therefore, the above carbohydrate is a disaccharide.
The carbohydrate shown in Figure 2 is an example of disaccharide.
(g)
Interpretation:
Whether glycogen is a monosaccharide, disaccharide, or polysaccharide is to be stated.
Concept introduction:
On the basis of the structural differences carbohydrates are classified into three parts.
Monosaccharide: They are the polyhydroxy aldehydes or ketones containing only one aldehyde or ketone unit. For example: glucose and fructose.
Disaccharide: They are the polyhydroxy aldehydes or ketones containing two aldehyde or ketone units. For example: Sucrose, lactose.
Polysaccharide: They are the polyhydroxy aldehydes or ketones containing more than two aldehyde or ketone units. For example: Cellulose, starch.
Answer to Problem 17.4E
Glycogen is a polysaccharide.
Explanation of Solution
Glycogen is a polymeric form of D-glucose which on hydrolysis gives glucose units. Each glucose unit is linked by
Glycogen on hydrolysis gives a large number of molecules of glucose. As a result, glycogen is a polysaccharide.
(h)
Interpretation:
Whether amulose is a monosaccharide, disaccharide, or polysaccharide is to be stated.
Concept introduction:
On the basis of the structural differences carbohydrates are classified into three parts.
Monosaccharide: They are the polyhydroxy aldehydes or ketones containing only one aldehyde or ketone unit. For example: glucose and fructose.
Disaccharide: They are the polyhydroxy aldehydes or ketones containing two aldehyde or ketone units. For example: Sucrose, lactose.
Polysaccharide: They are the polyhydroxy aldehydes or ketones containing more than two aldehyde or ketone units. For example: Cellulose, starch.
Answer to Problem 17.4E
Amylose is a polysaccharide.
Explanation of Solution
Amylose is a polymeric form of
Amylose on hydrolysis gives a large number of glucose units. As a result, amylose is a polysaccharide.
Want to see more full solutions like this?
Chapter 17 Solutions
Study Guide with Student Solutions Manual for Seager/Slabaugh/Hansen's Chemistry for Today: General, Organic, and Biochemistry, 9th Edition
- What is the reaction mechanism for this?arrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. + Drawing Arrows CH3ONA, CH3OH heat : Br:O Na → H H Br Na + H H H H H :0: .H + Undo Reset Done Q CH3 Drag To Pan +arrow_forwardWhat is the reaction mechanism for this?arrow_forward
- 20.19 Predict the structure of the major 1,2-addition product formed by reaction of one mole of Cl₂ with 3-methylenecyclohexene. Also predict the structure of the 1,4-addition product formed under these conditions. 20.20 Which of the two molecules shown do you expect to be the major product formed by 1,2-addition of HCI to cyclopentadiene? Explain. Cyclopentadiene + HC 3-Chlorocyclopentene (racemic) or 4-Chlorocyclopentene (racemic)arrow_forward20.35 Propose structural formulas for compounds A and B and specify the configuration of compound B. EtO₂C 250°C C14H2004 CO₂Et 1. Oso, then NaHSO3 2. HIO4 C14H2006 A Barrow_forward20.21 Predict the major product formed by 1,4-addition of HCI to cyclopentadiene. 20.22 Draw structural formulas for the two constitutional isomers with the molecular for- mula C₂H,Br, formed by adding one mole of Br, to cyclopentadiene.arrow_forward
- Add substituents to draw the conformer below (sighting down the indicated bond), then rotate the back carbon to provide the conformation that will be capable of an E2 elimination. R/S stereochemistry is graded. + I I H CH3 Ph Досн Br OCH 3 Drawing Q H Atoms, Bonds and Rings Charges Tap a node to see suggestions. H H H H H Undo Reset Remove Done Rotatearrow_forward20.17 Predict the structure of the major product formed by 1,2-addition of HBr to 3-methylenecyclohexene. 3-Methylenecyclohexene 20.18 Predict the major product formed by 1,4-addition of HBr to 3-methylenecyclohexene.arrow_forward+ Draw a vicinal alkyl bromide that would produce the following alkene in an E2 elimination. Use a dash or wedge bond to indicate stereochemistry on asymmetric centers, where applicable. Ignore any inorganic byproducts. Br Drawing Strong Base H Q Atoms, Bonds Charges and Rings Draw or tap a new bond to see suggestions. Remove Done 語 Reset Undo + Drag To Panarrow_forward
- Draw a vicinal alkyl bromide that would produce the following alkene in an E2 elimination. Use a dash or wedge bond to indicate stereochemistry on asymmetric centers, where applicable. Ignore any inorganic byproducts. + Drawing Į Strong Base H Br Q Atoms, Bonds and Rings Charges Draw or tap a new bond to see suggestions. Undo Reset 謂 Remove Done Drag To Pan +arrow_forwardDraw the product of the E2 reaction shown below. Include the correct stereochemistry. Ignore any inorganic byproducts. + Br CH3 Q Strong Base Drawing Atoms, Bonds and Rings Charges Undo Reset H "Br H N Br. Remove Done .N. Drag To Panarrow_forwardCurved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and follow the curved arrows to draw the product of this elementary step in an elimination mechanism. Include all lone pairs and charges as appropriate. Ignore stereochemistry. Ignore byproducts. + Br: .. 8 0.01 M NaOH heat Drawing Q Atoms, Bonds and Rings Charges and Lone Pairs Draw or tap a new bond to see suggestions. Undo Reset Remove Done + Drag To Panarrow_forward
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning





