
(a)
Interpretation: The structural formula for the given compound has to be drawn.
Concept introduction:
Carboxylic acids contain a carbonyl attached to a hydroxyl group as shown below,
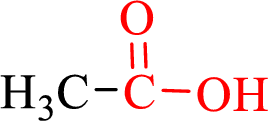
Nomenclature of
- • Find the Parent hydrocarbon chain.
- • Carboxyl carbon must be numbered first.
- • Replace the –e in the
alkane name with –oic acid.
Naming of compounds with two
If a compound has two functional groups, the one with lower priority is indicated by a prefix and another with the higher priority by a suffix.
Carboxylic salts are the water-soluble ammonium or alkali metal salts of carboxylic acids.
(a)
Explanation of Solution
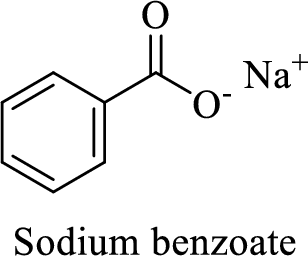
Name of the given salt is sodium benzoate.
From the name, we will get the following fact about the structure of the compound.
- ✓ The given compound is a sodium salt of benzoic acid.
Thus,
The structural formula for this salt can be drawn as shown below,
(b)
Interpretation: The structural formula for the given compound has to be drawn.
Concept introduction:
Carboxylic acids contain a carbonyl attached to a hydroxyl group as shown below,
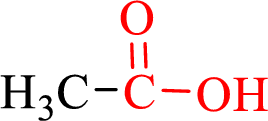
Nomenclature of carboxylic acid:
- • Find the Parent hydrocarbon chain.
- • Carboxyl carbon must be numbered first.
- • Replace the –e in the alkane name with –oic acid. If two or more carboxylic functional groups are present in the same compound then its number should be taken in to consideration and the prefix di, tri, tetra.. must be used.
Naming of compounds with two functional groups;
If a compound has two functional groups, the one with lower priority is indicated by a prefix and another with the higher priority by a suffix.
Carboxylic salts are the water-soluble ammonium or alkali metal salts of carboxylic acids.
(b)
Explanation of Solution
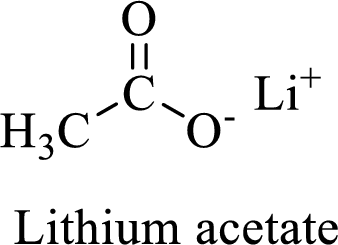
Name of the given salt is Lithium acetate.
From the name, we will get the following fact about the structure of the compound.
- ✓ The given compound is a Lithium salt of acetic acid
Thus,
The structural formula for this compound can be drawn as shown below,
(c)
Interpretation: The structural formula for the given compound has to be drawn.
Concept introduction:
Carboxylic acids contain a carbonyl attached to a hydroxyl group as shown below,
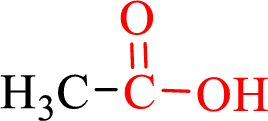
Nomenclature of carboxylic acid:
- • Find the Parent hydrocarbon chain.
- • Carboxyl carbon must be numbered first.
- • Replace the –e in the alkane name with –oic acid.
Naming of compounds with two functional groups;
If a compound has two functional groups, the one with lower priority is indicated by a prefix and another with the higher priority by a suffix.
Carboxylic salts are the water-soluble ammonium or alkali metal salts of carboxylic acids.
(c)
Explanation of Solution
Name of the given salt is Ammonium acetate.
From the name, we will get the following fact about the structure of the compound.
- ✓ The given compound is an ammonium salt of acetic acid.
Thus,
The structural formula for this compound can be drawn as shown below,
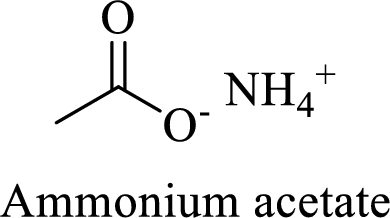
(d)
Interpretation: The structural formula for the given compound has to be drawn.
Concept introduction:
Carboxylic acids contain a carbonyl attached to a hydroxyl group as shown below,
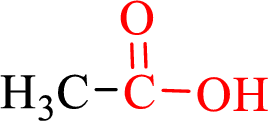
Nomenclature of carboxylic acid:
- • Find the Parent hydrocarbon chain.
- • Carboxyl carbon must be numbered first.
- • Replace the –e in the alkane name with –oic acid.
Naming of compounds with two functional groups;
If a compound has two functional groups, the one with lower priority is indicated by a prefix and another with the higher priority by a suffix.
Carboxylic salts are the water-soluble ammonium or alkali metal salts of carboxylic acids.
(d)
Explanation of Solution
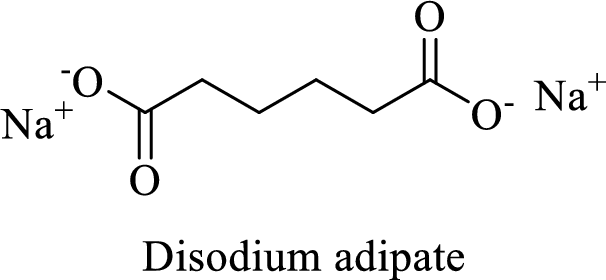
Name of the given salt is Disodium adipate.
From the name, we will get the following facts about the structure of the compound.
- ✓ The given compound is a sodium salt of adipic acid.
- ✓ Two sodium atoms are attached to the both carboxyl group of adipic acid.
The structure of adipic acid is shown below,
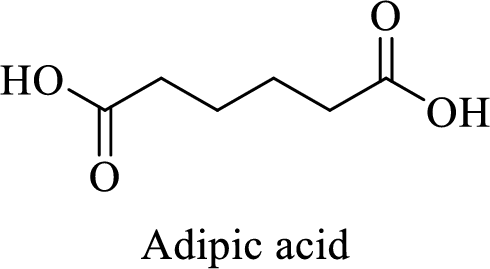
Thus,
The structural formula for this compound can be drawn as shown below,
(e)
Interpretation: The structural formula for the given compound has to be drawn.
Concept introduction:
Carboxylic acids contain a carbonyl attached to a hydroxyl group as shown below,
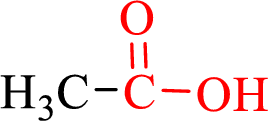
Nomenclature of carboxylic acid:
- • Find the Parent hydrocarbon chain.
- • Carboxyl carbon must be numbered first.
- • Replace the –e in the alkane name with –oic acid.
Naming of compounds with two functional groups;
If a compound has two functional groups, the one with lower priority is indicated by a prefix and another with the higher priority by a suffix.
Carboxylic salts are the water-soluble ammonium or alkali metal salts of carboxylic acids.
(e)
Explanation of Solution
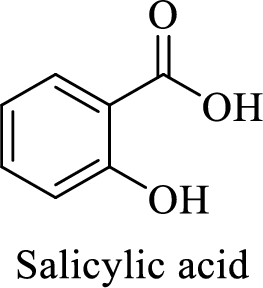
Name of the given salt is sodium salicylate.
From the name, we will get the following fact about the structure of the compound.
- ✓ The compound is a sodium salt of salicylic acid.
Structure of salicylic acid is shown below,
Thus,
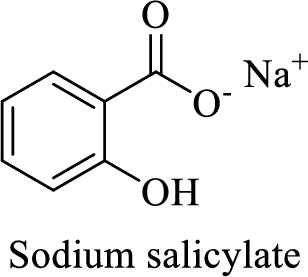
The structural formula for this compound can be drawn as shown below,
(f)
Interpretation: The structural formula for the given compound has to be drawn.
Concept introduction:
Carboxylic acids contain a carbonyl attached to a hydroxyl group as shown below,
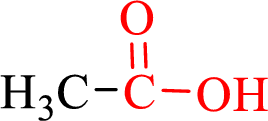
Nomenclature of carboxylic acid:
- • Find the Parent hydrocarbon chain.
- • Carboxyl carbon must be numbered first.
- • Replace the –e in the alkane name with –oic acid. If two or more carboxylic functional groups are present in the same compound then its number should be taken in to consideration and the prefix di, tri, tetra.. must be used.
Naming of compounds with two functional groups;
If a compound has two functional groups, the one with lower priority is indicated by a prefix and another with the higher priority by a suffix.
Carboxylic salts are the water-soluble ammonium or alkali metal salts of carboxylic acids.
(f)
Explanation of Solution
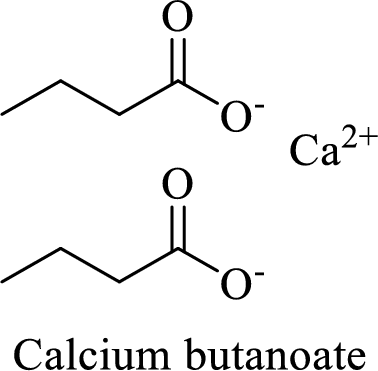
Name of the given salt is calcium butanoate.
From the name, we will get the following fact about the structure of the compound.
- ✓ The given compound is a calcium salt of butanoic acid.
Thus,
The structural formula for this compound can be drawn as shown below,
Want to see more full solutions like this?
Chapter 17 Solutions
Organic Chemistry, Loose-leaf Version
- Q8. Draw the mechanism for this halogenation reaction. Show all steps including initiation, propagation, and recombination. Cl₂, hv CI Br Br2, hv, heatarrow_forwardQ6. Given the following alkanes, draw the most likely product to form upon monohalogenation with Br2 (keep in mind that this may not be the only product to form though). If the reaction was performed with Cl2 would there be more or less selectivity in the desired product formation? Why? (a) (b) (c)arrow_forwardQ4. Radicals a. For the following indicated bonds, rank them in order of decreasing AH° for homolytic cleavage. Based on your answer, which bond would be most likely to break homolytically? (c) CH3 CH3 H3C CH3 (a) CH3 (b)arrow_forward
- Q1. (a) Draw equations for homolytic and heterolytic cleavages of the N-H bond in NH3. Use curved arrows to show the electron movement. (b) Draw equations for homolytic and heterolytic cleavages of the N-H bond in NH4*. Use curved arrows to show the electron movement.arrow_forwardohing Quantitative Relationships 425 The specific heats and atomic masses of 20 of the elements are given in the table below. Use a graphical method to determine if there is a relationship between specific heat and the atomic mass. a. b. C. d. e. If your graphs revealed relationship between specific heat and atomic revealed a mathematical mass, write down an equation for the relationship. Comment on the usefulness of the determination of specific heat as a method for identifying an element. Would specific heat alone give you much confidence with regard to the identity of the element? If you think measurement of another property would be needed to support an identification, what property would you measure and why? The elements listed in the table are all selected metals. The values for nitrogen, oxygen, fluorine and neon are 1.040, 0.918, 0.824 and 1.030 J/g K respectively. Do these elements fit your equation? element atomic mass specific heat (almol) (Jig K) magnesium 24.305 1.023…arrow_forwardPlease correct answer and don't use hand rating and don't use Ai solutionarrow_forward
- Nonearrow_forwardDraw Newman projects for each of the following molecules with 3 different rotational angles from carbon 2 to carbon 3. Rank your structures from lowest to highest energy. What causes the energy differences? Label the overlap. a. b. Br OH C. Br Brarrow_forwardDraw the stereoisomers of 3,5-diethylcylopentane. Identify the different relationships between each molecules (diasteromers, enantiomers, meso compounds, etc.)arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT

