
Concept explainers
(a)
Interpretation:
Show how to prepare pentanoic acid from 1-Pentanol.
Concept introduction:
Carboxylic acid on further oxidation removes the carboxyl carbon as carbon dioxide.
Depending on the reaction conditions, the oxidation state of the remaining organic structure may be higher, lower or unchanged.
Carboxylic acid can be prepared from primary alcohol by oxidation using strong oxidizing agents like chromic acid,
(b)
Interpretation:
Show how to prepare pentanoic acid from Pentanal.
Concept introduction:
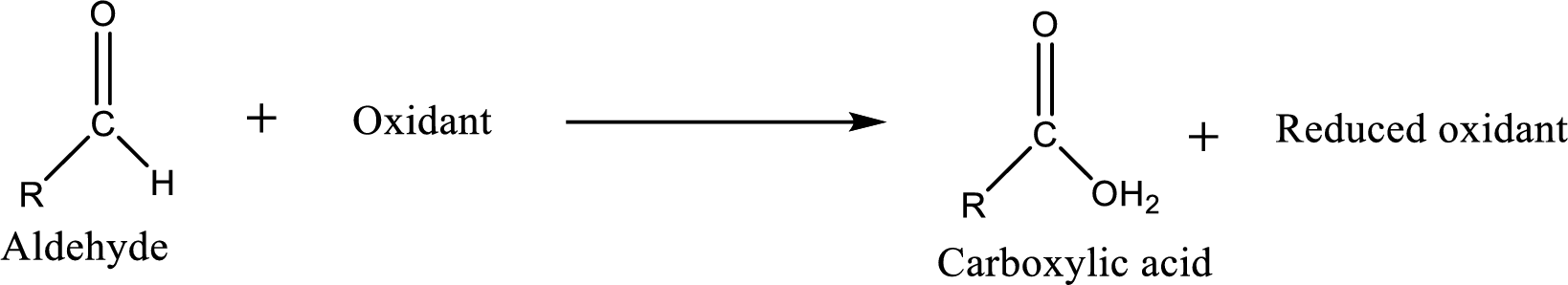
Carboxylic acid can be prepared from various ways; oxidation of aldehyde is one of the important methods to prepare carboxylic acid.
Aldehyde and
In aldehyde
In ketone
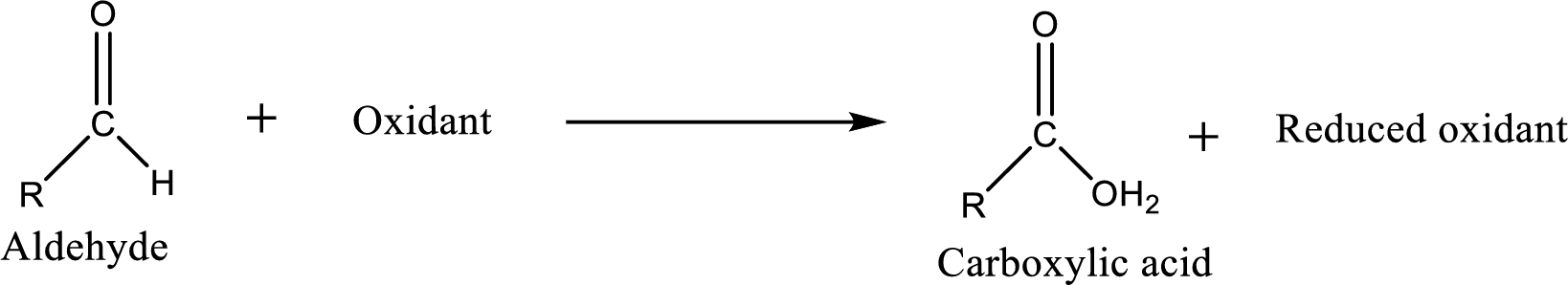
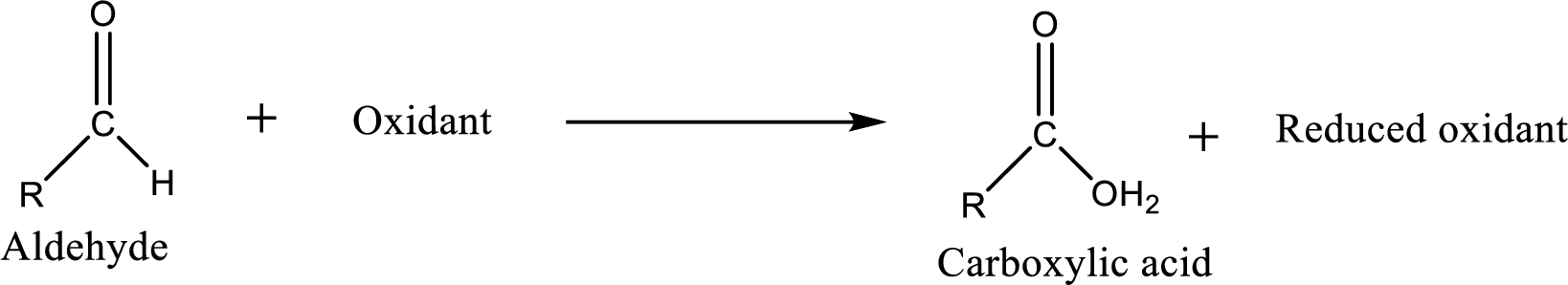
Aldehyde readily undergoes oxidation to carboxylic acids.
Tollens’ reagent is an ammoniac silver nitrate solution which can be used to detect the presence of aldehyde in an unknown compound.
As the oxidation of the aldehyde proceeds by Tollens’ reagent, silver metal is deposited on the walls of the reaction flask as a shiny mirror.
The reaction can be represented as follows,
(c)
Interpretation:
Show how to prepare pentanoic acid from 1-Pentene.
Concept introduction:
Carboxylic acid can be prepared from various ways; oxidation of aldehyde is one of the important methods to prepare carboxylic acid.
Aldehyde and ketones are one such an important group in the organic compounds. Both of these compounds contain carbonyl group
In aldehyde
In ketone
Carboxylic acid can be prepared from primary alcohol by oxidation using strong oxidizing agents like chromic acid,
Alkenes on acid catalyzed hydration will give alcohol.
(d)
Interpretation:
Show how to prepare pentanoic acid from 1-Butanol.
Concept introduction:
Alkyl or aryl magnesium halides (RMgX) are known as Grignard reagent. The Grignard reaction is an organometallic
Synthesis of Grignard reagent is shown below,
The
Addition of a Grignard reagent to carbon dioxide followed by protonation will produce carboxylic acid.
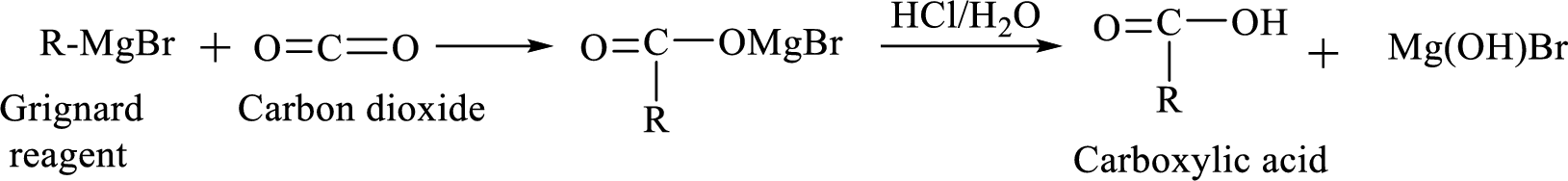
(e)
Interpretation:
Show how to prepare pentanoic acid from 1-Bromopropane.
Concept introduction:
Alkyl or aryl magnesium halides (RMgX) are known as Grignard reagent. The Grignard reaction is an organometallic chemical reaction in which the Grignard reagent act as nucleophile and attack electrophilic carbon atom that are present within polar bonds to yield a carbon-carbon bond.
Synthesis of Grignard reagent is shown below,
The alkyl halide can be prepared from alcohol through different methods, preparing alkyl halide using halogens is one of the important methods and it is shown below,
Addition of a Grignard reagent to carbon dioxide followed by protonation will produce carboxylic acid.
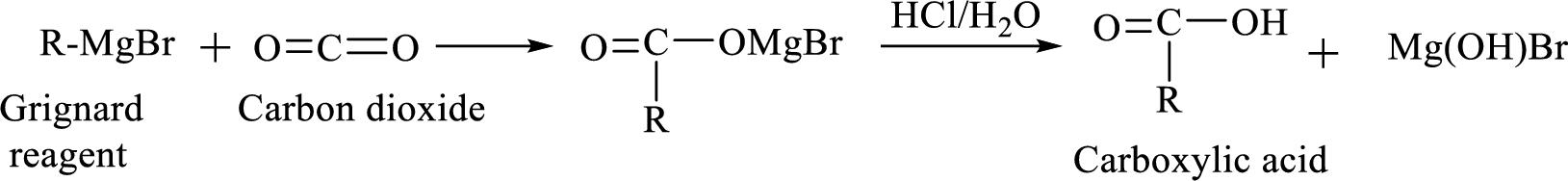
(f)
Interpretation:
Show how to prepare pentanoic acid from 1-Hexene.
Concept introduction:
Carboxylic acid can be prepared from various ways; oxidation of aldehyde is one of the important methods to prepare carboxylic acid.
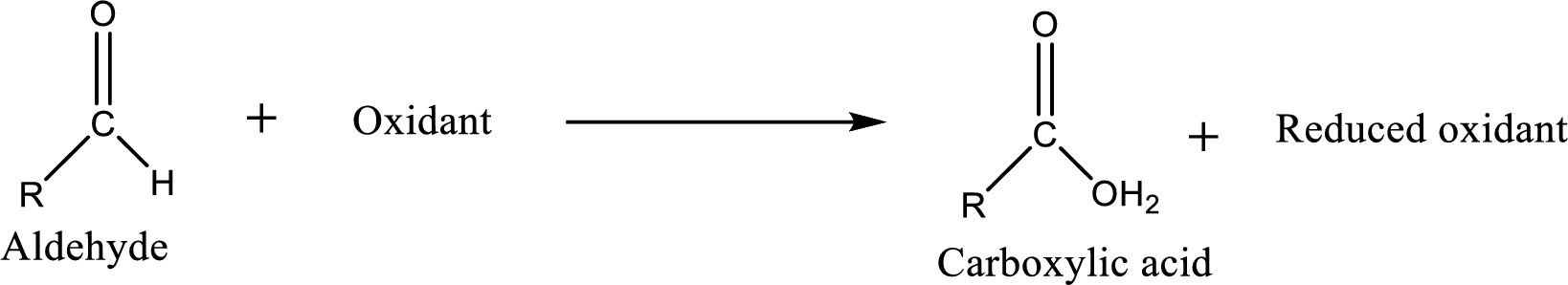
Aldehyde and ketones are one such an important group in the organic compounds. Both of these compounds contain carbonyl group
In aldehyde
In ketone
The reaction of
Trending nowThis is a popular solution!
Learn your wayIncludes step-by-step video

Chapter 17 Solutions
Organic Chemistry, Loose-leaf Version
- Can I get helpp drawing my arrowsarrow_forwardWhich of the m/z values corresponds to the base peak in the mass spectrum shown? 100 80 A. 45 B. 44 C. 29 D. 15 Intensity 20 0 10 20 30 40 B- m/z -8 50 E. 30 Which of the m/z values correspond to the molecular ion for the compound shown? A. 18 B. 82 OH C. 100 D. 102 E. 103arrow_forwardCan someone help me with drawing my arrows.arrow_forward
- I'm having trouble with converting lewis diagrams into VSEPR diagrams. I currently have this example of C2BrCl3 which I want to turn into a lewis structure, but I'm not sure what steps I need to do in order to do so. I have the table written down, however, there's two central atoms so what would I do? There seems to be 4 electron domains on the carbon atom and no lone pairs so it would seem like this shape would be tetrahedral. Here's what I have now. Thanks!arrow_forwardWe discussed the solid phase resin using in peptide synthesis. Provide a mechanism, for its formation. DRAW THE MECHANISM.arrow_forwardPlease help. Every time I've asked an expert in the past, it's been wrong :(arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





