
Concept explainers
(a)
Interpretation: The structural formula for the given compound has to be drawn.
Concept introduction:
Carboxylic acids contain a carbonyl attached to a hydroxyl group as shown below,
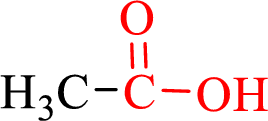
Nomenclature of
- • Find the Parent hydrocarbon chain.
- • Carboxyl carbon must be numbered first.
- • Replace the –e in the
alkane name with –oic acid.
Naming of compounds with two
If a compound has two functional groups, the one with lower priority is indicated by a prefix and another with the higher priority by a suffix.
(a)
Explanation of Solution
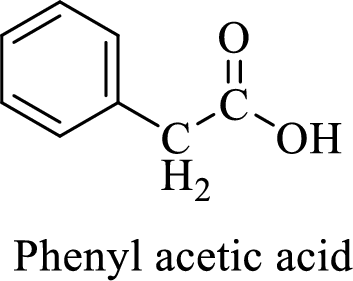
Name of the given compound is phenyl acetic acid.
From the name, we will get the following facts about the structure of the compound.
- ✓ The given compound is a carboxylic acid.
- ✓ A phenyl substituent is attached to the acetic acid; Acetic acid is the common name of ethanoic acid.
Thus,
The structural formula for this compound can be drawn as shown below,
(b)
Interpretation: The structural formula for the given compound has to be drawn.
Concept introduction:
Carboxylic acids contain a carbonyl attached to a hydroxyl group as shown below,
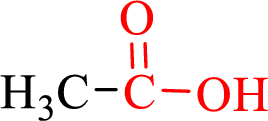
Nomenclature of carboxylic acid:
- • Find the Parent hydrocarbon chain.
- • Carboxyl carbon must be numbered first.
- • Replace the –e in the alkane name with –oic acid. If two or more carboxylic functional groups are present in the same compound then its number should be taken in to consideration and the prefix di, tri, tetra.. must be used.
Naming of compounds with two functional groups;
If a compound has two functional groups, the one with lower priority is indicated by a prefix and another with the higher priority by a suffix.
(b)
Explanation of Solution
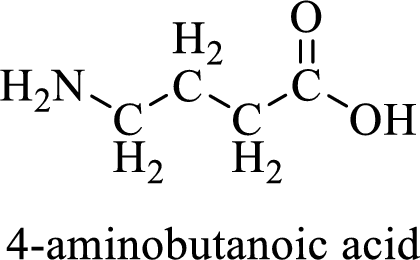
Name of the given compound is 4-Aminobutanoic acid
From the name, we will get the following facts about the structure of the compound.
- ✓ The given compound is a carboxylic acid.
- ✓ Parent chain contains four carbon atoms including the carboxylic acid functional group.
- ✓ An amino substituent is attached to the fourth carbon atom in the parent chain.
Thus,
The structural formula for this compound can be drawn as shown below,
(c)
Interpretation: The structural formula for the given compound has to be drawn.
Concept introduction:
Carboxylic acids contain a carbonyl attached to a hydroxyl group as shown below,
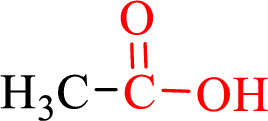
Nomenclature of carboxylic acid:
- • Find the Parent hydrocarbon chain.
- • Carboxyl carbon must be numbered first.
- • Replace the –e in the alkane name with –oic acid.
Naming of compounds with two functional groups;
If a compound has two functional groups, the one with lower priority is indicated by a prefix and another with the higher priority by a suffix.
(c)
Explanation of Solution
Name of the given compound is 3-Chloro-4-phenylbutanoic acid.
From the name, we will get the following facts about the structure of the compound.
- ✓ The given compound is a carboxylic acid.
- ✓ Parent chain contains four carbon atoms including the carboxylic acid functional group.
- ✓ One chlorine atom and a phenyl ring substituents are attached to the third and fourth carbon atoms in the parent chain respectively.
Thus,
The structural formula for this compound can be drawn as shown below,
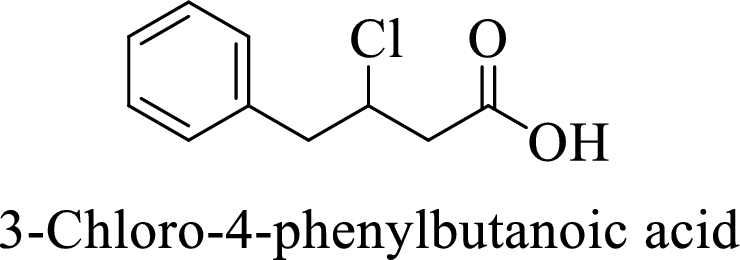
(d)
Interpretation: The structural formula for the given compound has to be drawn.
Concept introduction:
Carboxylic acids contain a carbonyl attached to a hydroxyl group as shown below,
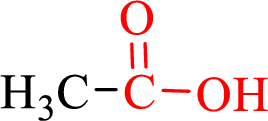
Nomenclature of carboxylic acid:
- • Find the Parent hydrocarbon chain.
- • Carboxyl carbon must be numbered first.
- • Replace the –e in the alkane name with –oic acid.
Naming of compounds with two functional groups;
If a compound has two functional groups, the one with lower priority is indicated by a prefix and another with the higher priority by a suffix.
(d)
Explanation of Solution
Name of the given compound is Propenoic acid (acrylic acid).
From the name, we will get the following facts about the structure of the compound.
- ✓ The given compound is a carboxylic acid.
- ✓ Parent chain contains three carbon atoms including the carboxylic acid functional group.
- ✓ There is a double bond in the compound.
Thus,
The structural formula for this compound can be drawn as shown below,
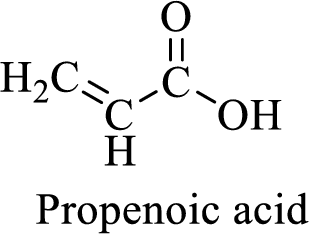
(e)
Interpretation: The structural formula for the given compound has to be drawn.
Concept introduction:
Carboxylic acids contain a carbonyl attached to a hydroxyl group as shown below,
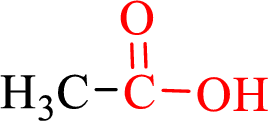
Nomenclature of carboxylic acid:
- • Find the Parent hydrocarbon chain.
- • Carboxyl carbon must be numbered first.
- • Replace the –e in the alkane name with –oic acid.
Naming of compounds with two functional groups;
If a compound has two functional groups, the one with lower priority is indicated by a prefix and another with the higher priority by a suffix.
E-Z designators are used as like cis-trans terminology for non-similar groups attached
In E-Z designations, the groups attached to vinylic positions are checked by their priority on the basis of higher molecular weight. If the higher priority groups are on the same sides, then the configuration is designated as Z. If the higher priority groups are on the opposite sides, then the configuration is designated as E.
(e)
Explanation of Solution
Name of the given compound is (Z)-3-Hexenedioic acid.
From the name, we will get the following facts about the structure of the compound.
- ✓ The given compound is a dicarboxylic acid.
- ✓ Parent chain contains six carbon atoms including the carboxylic acid functional group.
- ✓ There is a double bond in the compound. The higher priority substituents are on same side; (Z)
Thus,
The structural formula for this compound can be drawn as shown below,
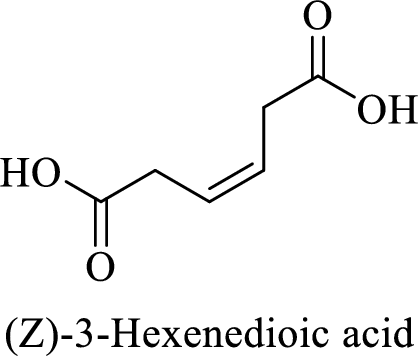
(f)
Interpretation: The structural formula for the given compound has to be drawn.
Concept introduction:
Carboxylic acids contain a carbonyl attached to a hydroxyl group as shown below,
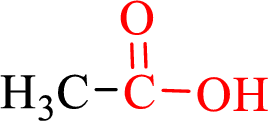
Nomenclature of carboxylic acid:
- • Find the Parent hydrocarbon chain.
- • Carboxyl carbon must be numbered first.
- • Replace the –e in the alkane name with –oic acid. If two or more carboxylic functional groups are present in the same compound then its number should be taken in to consideration and the prefix di, tri, tetra.. must be used.
Naming of compounds with two functional groups;
If a compound has two functional groups, the one with lower priority is indicated by a prefix and another with the higher priority by a suffix.
(f)
Explanation of Solution
Name of the given compound is 2-Pentynoic acid.
From the name, we will get the following facts about the structure of the compound.
- ✓ The given compound is a monocarboxylic acid.
- ✓ Parent chain contains five carbon atoms including the carboxylic acid functional group.
- ✓ There is a triple bond in between second and third carbon atoms in the compound.
Thus,
The structural formula for this compound can be drawn as shown below,
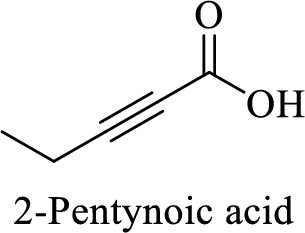
(g)
Interpretation: The structural formula for the given compound has to be drawn.
Concept introduction:
Carboxylic acids contain a carbonyl attached to a hydroxyl group as shown below,
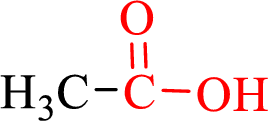
Nomenclature of carboxylic acid:
- • Find the Parent hydrocarbon chain.
- • Carboxyl carbon must be numbered first.
- • Replace the –e in the alkane name with –oic acid. If two or more carboxylic functional groups are present in the same compound then its number should be taken in to consideration and the prefix di, tri, tetra.. must be used.
Naming of compounds with two functional groups;
If a compound has two functional groups, the one with lower priority is indicated by a prefix and another with the higher priority by a suffix.
(g)
Explanation of Solution
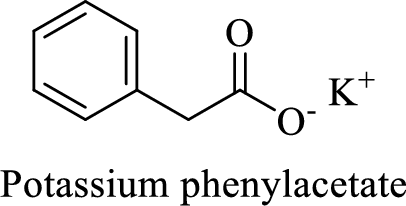
Name of the given compound is Potassium phenylacetate.
From the name, we will get the following facts about the structure of the compound.
- ✓ The given compound is a potassium salt of phenyl acetic acid.
- ✓ A phenyl substituent is attached to the acetic acid; Acetic acid is the common name of ethanoic acid.
Thus,
The structural formula for this compound can be drawn as shown below,
(h)
Interpretation: The structural formula for the given compound has to be drawn.
Concept introduction:
Carboxylic acids contain a carbonyl attached to a hydroxyl group as shown below,
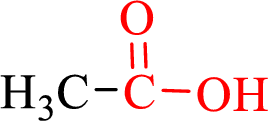
Nomenclature of carboxylic acid:
- • Find the Parent hydrocarbon chain.
- • Carboxyl carbon must be numbered first.
- • Replace the –e in the alkane name with –oic acid. If two or more carboxylic functional groups are present in the same compound then its number should be taken in to consideration and the prefix di, tri, tetra.. must be used.
Naming of compounds with two functional groups;
If a compound has two functional groups, the one with lower priority is indicated by a prefix and another with the higher priority by a suffix.
(h)
Explanation of Solution
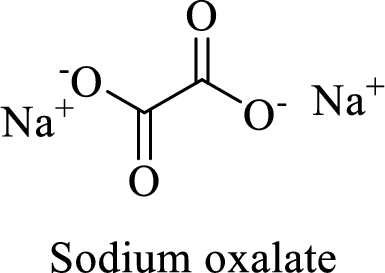
Name of the given compound is sodium oxalate.
From the name, we will get the following facts about the structure of the compound.
- ✓ The given compound is a sodium salt of oxalic acid.
- ✓ Two sodium atoms are attached to the both carboxyl group of oxalic acid.
Thus,
The structural formula for this compound can be drawn as shown below,
(i)
Interpretation: The structural formula for the given compound has to be drawn.
Concept introduction:
Carboxylic acids contain a carbonyl attached to a hydroxyl group as shown below,
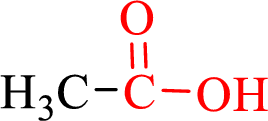
Nomenclature of carboxylic acid:
- • Find the Parent hydrocarbon chain.
- • Carboxyl carbon must be numbered first.
- • Replace the –e in the alkane name with –oic acid. If two or more carboxylic functional groups are present in the same compound then its number should be taken in to consideration and the prefix di, tri, tetra.. must be used.
Naming of compounds with two functional groups;
If a compound has two functional groups, the one with lower priority is indicated by a prefix and another with the higher priority by a suffix.
(i)
Explanation of Solution
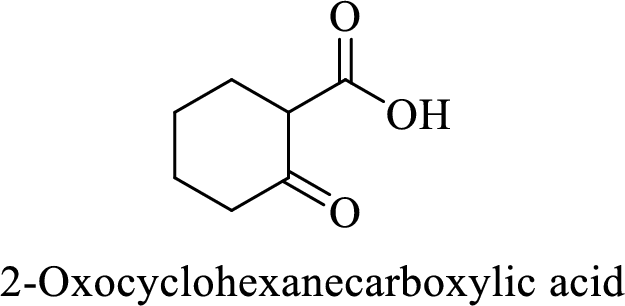
Name of the given compound is 2-Oxocyclohexanecarboxylic acid.
From the name, we will get the following facts about the structure of the compound.
- ✓ The given compound is a monocarboxylic acid.
- ✓ Parent ring contains six carbon atoms including the carboxylic acid functional group.
- ✓ There is a
ketone group on second carbon atom in the parent ring.
Thus,
The structural formula for this compound can be drawn as shown below,
(j)
Interpretation: The structural formula for the given compound has to be drawn.
Concept introduction:
Carboxylic acids contain a carbonyl attached to a hydroxyl group as shown below,
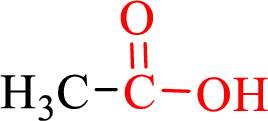
Nomenclature of carboxylic acid:
- • Find the Parent hydrocarbon chain.
- • Carboxyl carbon must be numbered first.
- • Replace the –e in the alkane name with –oic acid. If two or more carboxylic functional groups are present in the same compound then its number should be taken in to consideration and the prefix di, tri, tetra.. must be used.
Naming of compounds with two functional groups;
If a compound has two functional groups, the one with lower priority is indicated by a prefix and another with the higher priority by a suffix.
(j)
Explanation of Solution
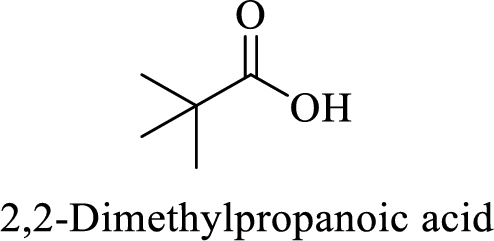
Name of the given compound is 2, 2-Dimethylpropanoic acid.
From the name, we will get the following facts about the structure of the compound.
- ✓ The given compound is a monocarboxylic acid.
- ✓ Parent chain contains three carbon atoms including the carboxylic acid functional group.
- ✓ Two methyl substituents are attached on the second carbon atom in the parent chain.
Thus,
The structural formula for this compound can be drawn as shown below,
Want to see more full solutions like this?
Chapter 17 Solutions
Organic Chemistry, Loose-leaf Version
- For each of the substituted benzene molecules below, determine the inductive and resonance effects the substituent will have on the benzene ring, as well as the overall electron-density of the ring compared to unsubstituted benzene. Molecule Inductive Effects Resonance Effects Overall Electron-Density × NO2 ○ donating O donating O withdrawing O withdrawing O electron-rich electron-deficient no inductive effects O no resonance effects O similar to benzene E [ CI O donating withdrawing O no inductive effects Explanation Check ○ donating withdrawing no resonance effects electron-rich electron-deficient O similar to benzene © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center Accesarrow_forwardUnderstanding how substituents activate Rank each of the following substituted benzene molecules in order of which will react fastest (1) to slowest (4) by electrophilic aromatic substitution. Explanation HN NH2 Check X (Choose one) (Choose one) (Choose one) (Choose one) © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center Aarrow_forwardIdentifying electron-donating and electron-withdrawing effects on benzene For each of the substituted benzene molecules below, determine the inductive and resonance effects the substituent will have on the benzene ring, as well as the overall electron-density of the ring compared to unsubstituted benzene. Inductive Effects Resonance Effects Overall Electron-Density Molecule CF3 O donating O donating O withdrawing O withdrawing O no inductive effects O no resonance effects electron-rich electron-deficient O similar to benzene CH3 O donating O withdrawing O no inductive effects O donating O withdrawing Ono resonance effects O electron-rich O electron-deficient O similar to benzene Explanation Check Х © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Centerarrow_forward
- * Hint: Think back to Chem 1 solubility rules. Follow Up Questions for Part B 12. What impact do the following disturbances to a system at equilibrium have on k, the rate constant for the forward reaction? Explain. (4 pts) a) Changing the concentration of a reactant or product. (2 pts) b) Changing the temperature of an exothermic reaction. (2 pts) ofarrow_forwardDraw TWO general chemical equation to prepare Symmetrical and non-Symmetrical ethers Draw 1 chemical reaction of an etherarrow_forwardPlease help me with the following questions for chemistry.arrow_forward
- + C8H16O2 (Fatty acid) + 11 02 → 8 CO2 a. Which of the above are the reactants? b. Which of the above are the products? H2o CO₂ c. Which reactant is the electron donor? Futty acid d. Which reactant is the electron acceptor? e. Which of the product is now reduced? f. Which of the products is now oxidized? 02 #20 102 8 H₂O g. Where was the carbon initially in this chemical reaction and where is it now that it is finished? 2 h. Where were the electrons initially in this chemical reaction and where is it now that it is finished?arrow_forward→ Acetyl-CoA + 3NAD+ + 1FAD + 1ADP 2CO2 + CoA + 3NADH + 1FADH2 + 1ATP a. Which of the above are the reactants? b. Which of the above are the products? c. Which reactant is the electron donor? d. Which reactants are the electron acceptors? e. Which of the products are now reduced? f. Which product is now oxidized? g. Which process was used to produce the ATP? h. Where was the energy initially in this chemical reaction and where is it now that it is finished? i. Where was the carbon initially in this chemical reaction and where is it now that it is finished? j. Where were the electrons initially in this chemical reaction and where is it now that it is finished?arrow_forwardRank each of the following substituted benzene molecules in order of which will react fastest (1) to slowest (4) by electrophilic aromatic substitution. OCH 3 (Choose one) OH (Choose one) Br (Choose one) Explanation Check NO2 (Choose one) © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Aarrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole

