
Connect 1-Semester Online Access for Principles of General, Organic & Biochemistry
2nd Edition
ISBN: 9780077633707
Author: Janice Smith
Publisher: Mcgraw-hill Higher Education (us)
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 16.3, Problem 16.6P
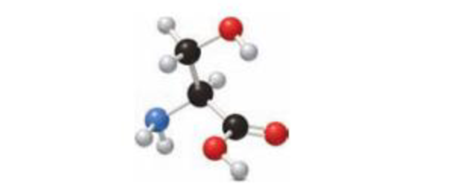
Identify the amino acid shown with all uncharged atoms in the ball-and-stick model, and draw the neutral, positively charged, and negatively charged forms of the amino acid.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Write the systematic (IUPAC) name for the following organic molecules.
Note for advanced students: you do not need to include any E or Z prefixes in your names.
Br
structure
Br
Br
Oweu
Conservation of mass was discussed in the background. Describe how conservation of mass (actual, not theoretical) could be checked in the experiment performed.
What impact would adding twice as much Na2CO3 than required for stoichiometric quantities have on the quantity of product produced? Initial results attached
Chapter 16 Solutions
Connect 1-Semester Online Access for Principles of General, Organic & Biochemistry
Ch. 16.2 - In addition to the amino and carboxyl groups, what...Ch. 16.2 - How do the OH groups in Ser, Thr, and Tyr differ?Ch. 16.2 - Draw both enantiomers of each amino acid in...Ch. 16.2 - Which of the following amino acids is naturally...Ch. 16.3 - Draw the structure of the amino acid valine at...Ch. 16.3 - Identify the amino acid shown with all uncharged...Ch. 16.3 - Draw the positively charged, neutral, and...Ch. 16.4 - Identify the N-terminal and C-terminal amino acid...Ch. 16.4 - (a) Identify the N-terminal amino acid in the...Ch. 16.4 - Identify the individual amino acids in each...
Ch. 16.4 - Prob. 16.11PCh. 16.5 - Prob. 16.12PCh. 16.6 - Prob. 16.13PCh. 16.6 - Draw the structures of each pair of amino acids...Ch. 16.6 - The fibroin proteins found in silk fibers consist...Ch. 16.7 - Prob. 16.16PCh. 16.7 - Prob. 16.17PCh. 16.8 - Prob. 16.18PCh. 16.8 - Prob. 16.19PCh. 16.8 - Prob. 16.20PCh. 16.9 - Prob. 16.21PCh. 16.9 - Prob. 16.22PCh. 16.9 - The nerve gas sarin acts as a poison by covalently...Ch. 16.10 - Prob. 16.24PCh. 16 - Prob. 16.25UKCCh. 16 - Prob. 16.26UKCCh. 16 - For each amino acid: [1] draw the L enantiomer in...Ch. 16 - For each amino acid: [1] give the name; [2] give...Ch. 16 - For the given tripeptide: (a) identify the amino...Ch. 16 - For the given tripeptide: (a) identify the amino...Ch. 16 - Label the regions of secondary structure in the...Ch. 16 - Label the regions of secondary structure in the...Ch. 16 - What type of interactions occur at each of the...Ch. 16 - Using the given representations for an enzyme and...Ch. 16 - Naturally occurring amino acids are L--amino...Ch. 16 - Why do neutral amino acids exist as zwitterions...Ch. 16 - The amino acid alanine is a solid at room...Ch. 16 - Why is phenylalanine water soluble but...Ch. 16 - Draw the structure of a naturally occurring amino...Ch. 16 - Draw the structure of a naturally occurring amino...Ch. 16 - For each amino acid: [1] draw the l enantiomer in...Ch. 16 - For each amino acid: [1] draw the l enantiomer in...Ch. 16 - Draw both enantiomers of each amino acid and label...Ch. 16 - Which of the following Fischer projections...Ch. 16 - For each amino acid: [1] give the name; [2] give...Ch. 16 - For each amino acid: [1] give the name; [2] give...Ch. 16 - Draw the amino acid leucine at each pH: (a) 6; (b)...Ch. 16 - Draw the amino acid isoleucine at each pH: (a) 6;...Ch. 16 - Draw the structure of the neutral, positively...Ch. 16 - Draw the structure of the neutral, positively...Ch. 16 - For each tripeptide: [1] identify the N-terminal...Ch. 16 - For each tripeptide: [1] identify the N-terminal...Ch. 16 - For the given tripeptide: (a) identify the amino...Ch. 16 - For the given tripeptide: (a) identify the amino...Ch. 16 - Locate the peptide bond in the dipeptide shown in...Ch. 16 - Label the N-terminal and C-terminal amino acids in...Ch. 16 - Draw the structures of the amino acids formed when...Ch. 16 - Draw the structures of the amino acids formed when...Ch. 16 - What amino acids are formed by hydrolysis of the...Ch. 16 - Give the three-letter abbreviations for the amino...Ch. 16 - What is the difference between the primary and...Ch. 16 - What is the difference between the tertiary and...Ch. 16 - What type of intermolecular forces exist between...Ch. 16 - Which of the following pairs of amino acids can...Ch. 16 - List two amino acids that would probably be...Ch. 16 - List two amino acids that would probably be...Ch. 16 - Compare -keratin and hemoglobin with regards to...Ch. 16 - Compare collagen and myoglobin with regards to...Ch. 16 - When a protein is denatured, how is its primary,...Ch. 16 - Hydrogen bonding stabilizes both the secondary and...Ch. 16 - Describe the function or biological activity of...Ch. 16 - Describe the function or biological activity of...Ch. 16 - Use the given representations for an enzyme,...Ch. 16 - Use the given representations for an enzyme and...Ch. 16 - How are enzyme inhibitors used to treat high blood...Ch. 16 - How are enzyme inhibitors used to treat HIV? Give...Ch. 16 - What structural feature in -keratin makes...Ch. 16 - Why does the -keratin in hair contain many...Ch. 16 - Why must vegetarian diets be carefully balanced?Ch. 16 - Why does cooking meat make it easier to digest?Ch. 16 - Sometimes an incision is cauterized (burned) to...Ch. 16 - Prob. 16.82APCh. 16 - How is sickle cell disease related to hemoglobin...Ch. 16 - The silk produced by a silkworm is a protein with...Ch. 16 - Explain the difference in the mechanism of action...Ch. 16 - How are blood enzyme levels used to diagnose...Ch. 16 - Explain why two amino acids aspartic acid and...Ch. 16 - Prob. 16.88CP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Given that a theoretical yield for isolating Calcium Carbonate in this experiment would be 100%. From that information and based on the results you obtained in this experiment, describe your success in the recovery of calcium carbonate and suggest two possible sources of error that would have caused you to not obtain 100% yield. Results are attached form experimentarrow_forward5) Calculate the flux of oxygen between the ocean and the atmosphere(2 pts), given that: (from Box 5.1, pg. 88 of your text): Temp = 18°C Salinity = 35 ppt Density = 1025 kg/m3 Oxygen concentration measured in bulk water = 263.84 mmol/m3 Wind speed = 7.4 m/s Oxygen is observed to be about 10% initially supersaturated What is flux if the temperature is 10°C ? (2 pts) (Hint: use the same density in your calculations). Why do your calculated values make sense (or not) based on what you know about the relationship between gas solubility and temperature (1 pt)?arrow_forwardFind a molecular formula for these unknownsarrow_forward
- (ME EX2) Prblms 8-11 Can you please explain problems 8 -11 to me in detail, step by step? Thank you so much! If needed color code them for me.arrow_forwardDon't used hand raitingarrow_forwardThe following 'H NMR spectrum was taken with a 750 MHz spectrometer: 1.0 0.5 0.0 10.0 9.0 8.0 7.0 6.0 5.0 4.0 3.0 ' 2.0 1.0 0.0 (ppm) What is the difference Av in the frequency of RF ac Δν ac radiation absorbed by the a and c protons? (Note: it's not equal to the difference in chemical shifts.) Round your answer to 2 significant digits, and be sure it has an appropriate unit symbol. = O O a will shift left, c will shift right. O a will shift right, c will shift left. a and c will both shift left, with more space between them. Suppose a new spectrum is taken with a 500 MHz spectrometer. What will be true about this new spectrum? O a and c will both shift left, with less space between them. O a and c will both shift right, with more space between them. O a and c will both shift right, with less space between them. Which protons have the largest energy gap between spin up and spin down states? O None of the above. ○ a Ob Explanation Check C Ar B 2025 McGraw Hill LLC. All Rights Reserved.…arrow_forward
- What mass of Na2CO3 must you add to 125g of water to prepare 0.200 m Na2CO3? Calculate mole fraction of Na2CO3, mass percent, and molarity of the resulting solution. MM (g/mol): Na2CO3 105.99; water 18.02. Final solution density is 1.04 g/mL.arrow_forward(ME EX2) Prblms Can you please explain problems to me in detail, step by step? Thank you so much! If needed color code them for me.arrow_forwardDon't used hand raitingarrow_forward
- Don't used hand raiting and don't used Ai solutionarrow_forwardFind a molecular formula. ( MW: 102 )arrow_forwardExperiment #8 Electrical conductivity & Electrolytes. Conductivity of solutions FLINN Scientific Scale RED LED Green LED LED Conductivity 0 OFF OFF 1 Dim OFF 2 medium OFF 3 Bright Dim Low or Nowe Low Medium High 4 Very Bright Medium nd very high AA Δ Δ Δ Δ Δ Δ Δ Δ Δ Δ Δ SE=Strong Electrolyte, FE = Fair Electrolyte CWE = Weak Electrolyte, NE= Noni Electrolyte, #Solutions 1 0.1 M NaCl 2/1x 102 M NaCl, 3/1X103 M Nall Can Prediction M Observed Conductivity Very bright red Bright red Dim red you help me understand how I'm supposed to find the predictions of the following solutions? I know this is an Ionic compound and that the more ions in a solution means it is able to carry a charge, right? AAAA Darrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div
World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

World of Chemistry
Chemistry
ISBN:9780618562763
Author:Steven S. Zumdahl
Publisher:Houghton Mifflin College Div

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning
Biomolecules - Protein - Amino acids; Author: Tutorials Point (India) Ltd.;https://www.youtube.com/watch?v=ySNVPDHJ0ek;License: Standard YouTube License, CC-BY