
Connect 1-Semester Online Access for Principles of General, Organic & Biochemistry
2nd Edition
ISBN: 9780077633707
Author: Janice Smith
Publisher: Mcgraw-hill Higher Education (us)
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 16, Problem 16.73AP
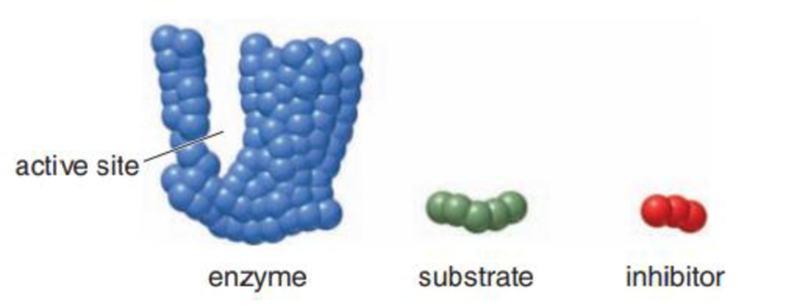
Use the given representations for an enzyme, substrate, and inhibitor to illustrate the process of noncompetitive inhibition.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Chapter 16 Solutions
Connect 1-Semester Online Access for Principles of General, Organic & Biochemistry
Ch. 16.2 - In addition to the amino and carboxyl groups, what...Ch. 16.2 - How do the OH groups in Ser, Thr, and Tyr differ?Ch. 16.2 - Draw both enantiomers of each amino acid in...Ch. 16.2 - Which of the following amino acids is naturally...Ch. 16.3 - Draw the structure of the amino acid valine at...Ch. 16.3 - Identify the amino acid shown with all uncharged...Ch. 16.3 - Draw the positively charged, neutral, and...Ch. 16.4 - Identify the N-terminal and C-terminal amino acid...Ch. 16.4 - (a) Identify the N-terminal amino acid in the...Ch. 16.4 - Identify the individual amino acids in each...
Ch. 16.4 - Prob. 16.11PCh. 16.5 - Prob. 16.12PCh. 16.6 - Prob. 16.13PCh. 16.6 - Draw the structures of each pair of amino acids...Ch. 16.6 - The fibroin proteins found in silk fibers consist...Ch. 16.7 - Prob. 16.16PCh. 16.7 - Prob. 16.17PCh. 16.8 - Prob. 16.18PCh. 16.8 - Prob. 16.19PCh. 16.8 - Prob. 16.20PCh. 16.9 - Prob. 16.21PCh. 16.9 - Prob. 16.22PCh. 16.9 - The nerve gas sarin acts as a poison by covalently...Ch. 16.10 - Prob. 16.24PCh. 16 - Prob. 16.25UKCCh. 16 - Prob. 16.26UKCCh. 16 - For each amino acid: [1] draw the L enantiomer in...Ch. 16 - For each amino acid: [1] give the name; [2] give...Ch. 16 - For the given tripeptide: (a) identify the amino...Ch. 16 - For the given tripeptide: (a) identify the amino...Ch. 16 - Label the regions of secondary structure in the...Ch. 16 - Label the regions of secondary structure in the...Ch. 16 - What type of interactions occur at each of the...Ch. 16 - Using the given representations for an enzyme and...Ch. 16 - Naturally occurring amino acids are L--amino...Ch. 16 - Why do neutral amino acids exist as zwitterions...Ch. 16 - The amino acid alanine is a solid at room...Ch. 16 - Why is phenylalanine water soluble but...Ch. 16 - Draw the structure of a naturally occurring amino...Ch. 16 - Draw the structure of a naturally occurring amino...Ch. 16 - For each amino acid: [1] draw the l enantiomer in...Ch. 16 - For each amino acid: [1] draw the l enantiomer in...Ch. 16 - Draw both enantiomers of each amino acid and label...Ch. 16 - Which of the following Fischer projections...Ch. 16 - For each amino acid: [1] give the name; [2] give...Ch. 16 - For each amino acid: [1] give the name; [2] give...Ch. 16 - Draw the amino acid leucine at each pH: (a) 6; (b)...Ch. 16 - Draw the amino acid isoleucine at each pH: (a) 6;...Ch. 16 - Draw the structure of the neutral, positively...Ch. 16 - Draw the structure of the neutral, positively...Ch. 16 - For each tripeptide: [1] identify the N-terminal...Ch. 16 - For each tripeptide: [1] identify the N-terminal...Ch. 16 - For the given tripeptide: (a) identify the amino...Ch. 16 - For the given tripeptide: (a) identify the amino...Ch. 16 - Locate the peptide bond in the dipeptide shown in...Ch. 16 - Label the N-terminal and C-terminal amino acids in...Ch. 16 - Draw the structures of the amino acids formed when...Ch. 16 - Draw the structures of the amino acids formed when...Ch. 16 - What amino acids are formed by hydrolysis of the...Ch. 16 - Give the three-letter abbreviations for the amino...Ch. 16 - What is the difference between the primary and...Ch. 16 - What is the difference between the tertiary and...Ch. 16 - What type of intermolecular forces exist between...Ch. 16 - Which of the following pairs of amino acids can...Ch. 16 - List two amino acids that would probably be...Ch. 16 - List two amino acids that would probably be...Ch. 16 - Compare -keratin and hemoglobin with regards to...Ch. 16 - Compare collagen and myoglobin with regards to...Ch. 16 - When a protein is denatured, how is its primary,...Ch. 16 - Hydrogen bonding stabilizes both the secondary and...Ch. 16 - Describe the function or biological activity of...Ch. 16 - Describe the function or biological activity of...Ch. 16 - Use the given representations for an enzyme,...Ch. 16 - Use the given representations for an enzyme and...Ch. 16 - How are enzyme inhibitors used to treat high blood...Ch. 16 - How are enzyme inhibitors used to treat HIV? Give...Ch. 16 - What structural feature in -keratin makes...Ch. 16 - Why does the -keratin in hair contain many...Ch. 16 - Why must vegetarian diets be carefully balanced?Ch. 16 - Why does cooking meat make it easier to digest?Ch. 16 - Sometimes an incision is cauterized (burned) to...Ch. 16 - Prob. 16.82APCh. 16 - How is sickle cell disease related to hemoglobin...Ch. 16 - The silk produced by a silkworm is a protein with...Ch. 16 - Explain the difference in the mechanism of action...Ch. 16 - How are blood enzyme levels used to diagnose...Ch. 16 - Explain why two amino acids aspartic acid and...Ch. 16 - Prob. 16.88CP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- How is enzyme specificity explained by the lock-and-key theory?arrow_forwardThe process by which an enzyme acts on the substrate can be described by the: a. lock-and-key model. b. enzyme-and-substrate model. c. enzyme folding model. d. catalytic model.arrow_forwardDraw a graph that shows the effect of increasing substrate concentration on the rate of an enzyme-catalyzed reaction (at constant temperature, pH, and enzyme concentration).arrow_forward
- Which of the following is not a characteristic of enzymes? a. They are macromolecules. b. They act on substances. c. They are phospholipids. d. They initiate and decelerate chemical reactions. e. They act as catalysts.arrow_forwardThe human body has an average pH of about 7 and a temperature of about 37C. Use graphs to illustrate enzyme activity in the human body as a function of the following: a. Substrate concentration b. Enzyme concentration c. pH include pH optimum value d. Temperature include temperature optimum valuearrow_forwardWhen handling or storing solutions of enzymes, the pH is usually kept near 7.0. Explain why.arrow_forward
- Write a brief description of the relationships among each of the following groups of terms or phrases. Answers to the Concept-Linking Exercises are given at the end of the chapter. Enzyme, enzyme substrate, active site, induced fit modelarrow_forwardBased on the graphical information in Problem 21-41 about enzymes A and B indicate whether the enzyme activity of enzyme B increases or decreases when the following changes in reaction conditions are made. a. pH decreases from 7.6 to 7.2 b. pH increases from 7.2 to 7.4 c. temperature decreases from 37.8C to 37.6C d. temperature increases from 38.2C to 38.4Carrow_forwardWhich is NOT a characteristic of proteins? a. They contain genetic information. b. They can act as hormones. c. They can catalyze chemical reactions. d. They act in cell membrane trafficking.arrow_forward
- 21-52 What are the chemical and physiological functions of the COX-2 enzyme?arrow_forwardIndicate whether each of the following statements describes a reversible competitive inhibitor, a reversible noncompetitive inhibitor, or an irreversible inhibitor. More than one answer may apply. a. It bonds covalently to the enzyme active site. b. The inhibitor effect can be reversed by the addition of more substrate. c. Inhibitor structure must be somewhat similar to that of the substrate. d. The inhibitor and substrate cannot bind to the enzyme simultaneously.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div
World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

World of Chemistry
Chemistry
ISBN:9780618562763
Author:Steven S. Zumdahl
Publisher:Houghton Mifflin College Div
DIGESTER-35 | VITAMINS AND THEIR RELATED COENZYMES| GPAT | NIPER | PHARMACIST| DI; Author: GPAT DISCUSSION CENTER;https://www.youtube.com/watch?v=CGrdNYmho0s;License: Standard YouTube License, CC-BY