
Connect 1-Semester Online Access for Principles of General, Organic & Biochemistry
2nd Edition
ISBN: 9780077633707
Author: Janice Smith
Publisher: Mcgraw-hill Higher Education (us)
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 16, Problem 16.74AP
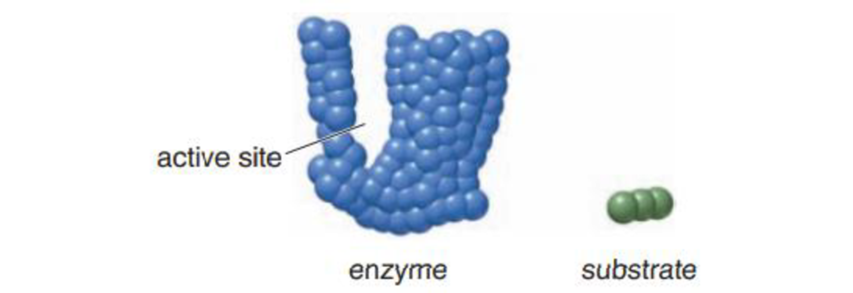
Use the given representations for an enzyme and substrate to illustrate the difference between the lock-and-key model and the induced-fit model of enzyme specificity.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Draw the major product of
this reaction. Ignore
inorganic
byproducts.
Incorrect, 1 attempt
remaining
1. LiAlH4
2. H3O+
Q
OH
☑
Select to Draw
How should I graph my data for the Absorbance of Pb and Fe for each mushroom? I want to compare the results to the known standard curve.
Software: Excel Spreadsheets
Link: https://mnscu-my.sharepoint.com/:x:/g/personal/vi2163ss_go_minnstate_edu/Eb2PfHdfEtBJiWh0ipHZ_kkBW4idWWwvpLPPtqoq2WkgbQ?rtime=HxrF0_tR3Ug
Provide the proper IUPAC name only for the following
compound. Dashes, commas, and spaces must be used
correctly, but do not use italics in Canvas.
Chapter 16 Solutions
Connect 1-Semester Online Access for Principles of General, Organic & Biochemistry
Ch. 16.2 - In addition to the amino and carboxyl groups, what...Ch. 16.2 - How do the OH groups in Ser, Thr, and Tyr differ?Ch. 16.2 - Draw both enantiomers of each amino acid in...Ch. 16.2 - Which of the following amino acids is naturally...Ch. 16.3 - Draw the structure of the amino acid valine at...Ch. 16.3 - Identify the amino acid shown with all uncharged...Ch. 16.3 - Draw the positively charged, neutral, and...Ch. 16.4 - Identify the N-terminal and C-terminal amino acid...Ch. 16.4 - (a) Identify the N-terminal amino acid in the...Ch. 16.4 - Identify the individual amino acids in each...
Ch. 16.4 - Prob. 16.11PCh. 16.5 - Prob. 16.12PCh. 16.6 - Prob. 16.13PCh. 16.6 - Draw the structures of each pair of amino acids...Ch. 16.6 - The fibroin proteins found in silk fibers consist...Ch. 16.7 - Prob. 16.16PCh. 16.7 - Prob. 16.17PCh. 16.8 - Prob. 16.18PCh. 16.8 - Prob. 16.19PCh. 16.8 - Prob. 16.20PCh. 16.9 - Prob. 16.21PCh. 16.9 - Prob. 16.22PCh. 16.9 - The nerve gas sarin acts as a poison by covalently...Ch. 16.10 - Prob. 16.24PCh. 16 - Prob. 16.25UKCCh. 16 - Prob. 16.26UKCCh. 16 - For each amino acid: [1] draw the L enantiomer in...Ch. 16 - For each amino acid: [1] give the name; [2] give...Ch. 16 - For the given tripeptide: (a) identify the amino...Ch. 16 - For the given tripeptide: (a) identify the amino...Ch. 16 - Label the regions of secondary structure in the...Ch. 16 - Label the regions of secondary structure in the...Ch. 16 - What type of interactions occur at each of the...Ch. 16 - Using the given representations for an enzyme and...Ch. 16 - Naturally occurring amino acids are L--amino...Ch. 16 - Why do neutral amino acids exist as zwitterions...Ch. 16 - The amino acid alanine is a solid at room...Ch. 16 - Why is phenylalanine water soluble but...Ch. 16 - Draw the structure of a naturally occurring amino...Ch. 16 - Draw the structure of a naturally occurring amino...Ch. 16 - For each amino acid: [1] draw the l enantiomer in...Ch. 16 - For each amino acid: [1] draw the l enantiomer in...Ch. 16 - Draw both enantiomers of each amino acid and label...Ch. 16 - Which of the following Fischer projections...Ch. 16 - For each amino acid: [1] give the name; [2] give...Ch. 16 - For each amino acid: [1] give the name; [2] give...Ch. 16 - Draw the amino acid leucine at each pH: (a) 6; (b)...Ch. 16 - Draw the amino acid isoleucine at each pH: (a) 6;...Ch. 16 - Draw the structure of the neutral, positively...Ch. 16 - Draw the structure of the neutral, positively...Ch. 16 - For each tripeptide: [1] identify the N-terminal...Ch. 16 - For each tripeptide: [1] identify the N-terminal...Ch. 16 - For the given tripeptide: (a) identify the amino...Ch. 16 - For the given tripeptide: (a) identify the amino...Ch. 16 - Locate the peptide bond in the dipeptide shown in...Ch. 16 - Label the N-terminal and C-terminal amino acids in...Ch. 16 - Draw the structures of the amino acids formed when...Ch. 16 - Draw the structures of the amino acids formed when...Ch. 16 - What amino acids are formed by hydrolysis of the...Ch. 16 - Give the three-letter abbreviations for the amino...Ch. 16 - What is the difference between the primary and...Ch. 16 - What is the difference between the tertiary and...Ch. 16 - What type of intermolecular forces exist between...Ch. 16 - Which of the following pairs of amino acids can...Ch. 16 - List two amino acids that would probably be...Ch. 16 - List two amino acids that would probably be...Ch. 16 - Compare -keratin and hemoglobin with regards to...Ch. 16 - Compare collagen and myoglobin with regards to...Ch. 16 - When a protein is denatured, how is its primary,...Ch. 16 - Hydrogen bonding stabilizes both the secondary and...Ch. 16 - Describe the function or biological activity of...Ch. 16 - Describe the function or biological activity of...Ch. 16 - Use the given representations for an enzyme,...Ch. 16 - Use the given representations for an enzyme and...Ch. 16 - How are enzyme inhibitors used to treat high blood...Ch. 16 - How are enzyme inhibitors used to treat HIV? Give...Ch. 16 - What structural feature in -keratin makes...Ch. 16 - Why does the -keratin in hair contain many...Ch. 16 - Why must vegetarian diets be carefully balanced?Ch. 16 - Why does cooking meat make it easier to digest?Ch. 16 - Sometimes an incision is cauterized (burned) to...Ch. 16 - Prob. 16.82APCh. 16 - How is sickle cell disease related to hemoglobin...Ch. 16 - The silk produced by a silkworm is a protein with...Ch. 16 - Explain the difference in the mechanism of action...Ch. 16 - How are blood enzyme levels used to diagnose...Ch. 16 - Explain why two amino acids aspartic acid and...Ch. 16 - Prob. 16.88CP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The kinetics of a gas phase reaction of the form A → Products results in a rate constant of 0.00781 M/min. For this reaction, the initial concentration of A is 0.501 M. How many minutes will it take for the concentration of A to reach 0.144 Marrow_forwardWhat is the rate for the second order reaction A → Products when [A] = 0.256 M? (k = 0.761 M⁻¹s⁻¹)arrow_forwardFor reaction N2(g) + O2(g) --> 2NO(g) Write the rate of the reaction in terms of change of NO.arrow_forward
- What is the name of the major product formed during the reaction between benzoyl chloride and phenol? benzyl ester O phenyl benzoate ○ cyclopentanoate ○ benzyl phenoate ○ benzenecarboxylic acidarrow_forwardProvide the proper IUPAC or common name for the following compound. Dashes, commas, and spaces must be used correctly.arrow_forwardProvide the proper IUPAC name (only) for the following compound. Dashes, commas, and spaces must be used correctly. HO. OHarrow_forward
- Question 2 0/1 pts Provide the proper IUPAC name only for the following compound. Dashes, commas, and spaces must be used correctly. HO CH 3 1-methyl-1-cyclohexanecarboxylic acidarrow_forwardPlease assign all the carbons for C-NMR and hydrogen for H-NMR. Please if I can get that less than hourarrow_forwardAssign these peaks spectrum ( H-NMR and C-NMR)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div
World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

World of Chemistry
Chemistry
ISBN:9780618562763
Author:Steven S. Zumdahl
Publisher:Houghton Mifflin College Div

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning
Biomolecules - Protein - Amino acids; Author: Tutorials Point (India) Ltd.;https://www.youtube.com/watch?v=ySNVPDHJ0ek;License: Standard YouTube License, CC-BY