
Chemistry: Principles and Reactions
8th Edition
ISBN: 9781305079373
Author: William L. Masterton, Cecile N. Hurley
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 16, Problem 90QAP
Consider the graph below: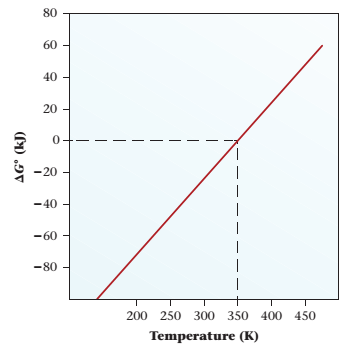
(a) Describe the relationship between the spontaneity of the process and temperature.
(b) Is the reaction exothermic?
(c)
(d) At what temperature is the reaction at standard conditions likely to be at equilibrium?
(e) Estimate K for the reaction at 97°C.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
10. The main product of the following reaction is [1.1:4',1"-terphenyl]-2'-yl(1h-pyrazol-4-
yl)methanone
Ph
N-H
Ph
Draw the Fischer projection for a D-aldo-pentose. (aldehyde pentose). How many total
stereoisomers are there? Name the sugar you drew.
Draw the Fischer projection for a L-keto-hexose. (ketone pentose). How many total
stereoisomers are there? Draw the enantiomer.
Draw a structure using wedges and dashes for the following compound:
H-
Et
OH
HO-
H
H-
Me
OH
Chapter 16 Solutions
Chemistry: Principles and Reactions
Ch. 16 - Spontaneous Processes Which of the following...Ch. 16 - Which of the following processes are spontaneous?...Ch. 16 - Which of the following processes are spontaneous?...Ch. 16 - Which of the following processes are spontaneous?...Ch. 16 - On the basis of your experience, predict which...Ch. 16 - On the basis of your experience, predict which of...Ch. 16 - In each of the following pairs, choose the...Ch. 16 - In each of the following pairs, choose the...Ch. 16 - Predict the sign of ΔS for the following: (a) a...Ch. 16 - Predict the sign of S for the following: (a)...
Ch. 16 - Predict the sign of S for each of the following...Ch. 16 - Predict the sign of S for each of the following...Ch. 16 - Predict the sign of S for each of the following...Ch. 16 - Predict the sign of S for each of the following...Ch. 16 - Predict the order of the following reactions in...Ch. 16 - Predict the order of the following reactions in...Ch. 16 - Use Table 16.1 to calculate S for each of the...Ch. 16 - Prob. 18QAPCh. 16 - Use Table 16.1 to calculate S for each of the...Ch. 16 - Use Table 16.1 to calculate S for each of the...Ch. 16 - Prob. 21QAPCh. 16 - Prob. 22QAPCh. 16 - Calculate G at 82C for reactions in which (a)...Ch. 16 - Calculate G at 72C for reactions in which (a)...Ch. 16 - Calculate G at 355 K for each of the reactions in...Ch. 16 - Calculate G at 415 K for each of the reactions in...Ch. 16 - From the values for G f given in Appendix 1,...Ch. 16 - Follow the directions of Problem 27 for each of...Ch. 16 - Use standard entropies and heats of formation to...Ch. 16 - Follow the directions of Question 29 for the...Ch. 16 - It has been proposed that wood alcohol, CH3OH, a...Ch. 16 - Prob. 32QAPCh. 16 - Sodium carbonate, also called washing soda, can be...Ch. 16 - The reaction between magnesium metal and water (l)...Ch. 16 - In the laboratory, POCl3 (phosphorus oxychloride)...Ch. 16 - Oxygen can be made in the laboratory by reacting...Ch. 16 - Phosgene, COCl2, can be formed by the reaction of...Ch. 16 - When permanganate ions in aqueous solution react...Ch. 16 - Discuss the effect of temperature change on the...Ch. 16 - Discuss the effect of temperature on the...Ch. 16 - At what temperature does G become zero for each of...Ch. 16 - Over what temperature range are the reactions in...Ch. 16 - For the reaction...Ch. 16 - For the reaction...Ch. 16 - For the decomposition of Ag2O:...Ch. 16 - Consider the following hypothetical equation...Ch. 16 - Prob. 47QAPCh. 16 - Prob. 48QAPCh. 16 - Red phosphorus is formed by heating white...Ch. 16 - Organ pipes in unheated churches develop tin...Ch. 16 - Prob. 51QAPCh. 16 - Pencil lead is almost pure graphite. Graphite is...Ch. 16 - Given the following data for sodium Na(s): S =51.2...Ch. 16 - Given the following data for bromine: Br2(l); S...Ch. 16 - Show by calculation, using Appendix 1, whether...Ch. 16 - Show by calculation whether the reaction HF(aq)...Ch. 16 - For the reaction...Ch. 16 - For the reaction...Ch. 16 - Consider the reaction 2SO2(g)+O2(g)2SO3(g) (a)...Ch. 16 - Consider the reaction AgCl(s)Ag+(aq)+Cl(aq) (a)...Ch. 16 - Consider the reaction CO(g)+H2O(g)CO2(g)+H2(g) Use...Ch. 16 - Consider the reaction NH4+(aq) H+(aq)+NH3(aq) Use ...Ch. 16 - Consider the following reaction at 25C:...Ch. 16 - Consider the reaction N2O(g)+NO2(g)3NO(g)K=4.41019...Ch. 16 - For the reaction...Ch. 16 - Consider the decomposition of N2O4 at 100C....Ch. 16 - Use the values for G f in Appendix 1 to calculate...Ch. 16 - Given that H f for HF(aq) is -320.1 kJ/mol and S...Ch. 16 - At 25C, a 0.327 M solution of a weak acid HX has a...Ch. 16 - A 0.250 M solution of a weak base R2NH has a pH of...Ch. 16 - Prob. 71QAPCh. 16 - Given the following standard free energies at 25°C...Ch. 16 - Natural gas, which is mostly methane, CH4, is a...Ch. 16 - Prob. 74QAPCh. 16 - When glucose, C6H12O11, is metabolized to CO2 and...Ch. 16 - Consider the following reactions at 25°C:...Ch. 16 - At 1200 K, an equilibrium mixture of CO and CO2...Ch. 16 - Prob. 78QAPCh. 16 - Prob. 79QAPCh. 16 - Prob. 80QAPCh. 16 - Prob. 81QAPCh. 16 - Carbon monoxide poisoning results when carbon...Ch. 16 - Prob. 83QAPCh. 16 - Determine whether each of the following statements...Ch. 16 - Which of the following quantities can be taken to...Ch. 16 - Fill in the blanks: (a) H° and G° become equal at...Ch. 16 - Fill in the blanks: (a) At equilibrium, G is. (b)...Ch. 16 - Prob. 88QAPCh. 16 - Consider the following reaction with its...Ch. 16 - Consider the graph below: (a) Describe the...Ch. 16 - Prob. 91QAPCh. 16 - Prob. 92QAPCh. 16 - Prob. 93QAPCh. 16 - Hf for iodine gas is 62.4 kJ/mol, and S° is 260.7...Ch. 16 - Prob. 95QAPCh. 16 - The overall reaction that occurs when sugar is...Ch. 16 - Hydrogen has been suggested as the fuel of the...Ch. 16 - When a copper wire is exposed to air at room...Ch. 16 - Kafor acetic acid (HC2H3O2) at 25°C is 1.754105 ....Ch. 16 - Consider the reaction 2HI(g)H2(g)+I2(g)At 500C a...Ch. 16 - Prob. 101QAPCh. 16 - Consider the formation of HI(g) from H2(g) and...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which of the following molecules are NOT typical carbohydrates? For the molecules that are carbohydrates, label them as an aldose or ketose. HO Он ОН ОН Он ОН но ΤΗ HO ОН HO eve Он он ОН ОН ОН If polyethylene has an average molecular weight of 25,000 g/mol, how many repeat units are present?arrow_forwardDraw the a-anomer cyclized pyranose Haworth projection of the below hexose. Circle the anomeric carbons. Number the carbons on the Fischer and Haworth projections. Assign R and S for each chiral center. HO CHO -H HO -H H- -OH H -OH CH₂OH Draw the ẞ-anomer cyclized furanose Haworth projection for the below hexose. Circle the anomeric carbons. Number the carbons on the Fischer and Haworth projections. HO CHO -H H -OH HO -H H -OH CH₂OHarrow_forwardName the below disaccharide. Circle any hemiacetals. Identify the numbering of glycosidic linkage, and identify it as a or ẞ. OH HO HO OH HO HO HO OHarrow_forward
- What are the monomers used to make the following polymers? F. а. b. с. d. Вецер хочому なarrow_forward1. Propose a reasonable mechanism for the following transformation. I'm looking for curved mechanistic arrows and appropriate formal charges on intermediates. OMe MeO OMe Me2N NMe2 OTBS OH xylenes OMe 'OTBSarrow_forwardWhat is the polymer made from the following monomers? What type of polymerization is used for each? а. ОН H2N но b. ن -NH2 d. H₂N NH2 довarrow_forward
- Condensation polymers are produced when monomers containing two different functional groups link together with the loss of a small molecule such as H2O. The difunctional monomer H2N(CH2)6COOH forms a condensation polymer. Draw the carbon-skeleton structure of the dimer that forms from this monomer.arrow_forwardWhat is the structure of the monomer?arrow_forward→ BINDERIYA GANBO... BINDERIYA GANBO. AP Biology Notes Gamino acid chart - G... 36:22 司 10 ☐ Mark for Review Q 1 Hide 80 8 2 =HA O=A¯ = H₂O Acid HIO HBrO HCIO Question 10 of 35 ^ Σ DELL □ 3 % Λ & 6 7 * ∞ 8 do 5 $ 4 # m 3 ° ( 9 Highlights & Notes AXC Sign out Carrow_forward
- Which representation(s) show polymer structures that are likely to result in rigid, hard materials and those that are likely to result in flexible, stretchable, soft materials?arrow_forward3. Enter the molecular weight of the product obtained from the Williamson Ether Synthesis? OH OH & OH excess CH3l Ag₂Oarrow_forwardPlease answer 1, 2 and 3 on the endarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning

The Laws of Thermodynamics, Entropy, and Gibbs Free Energy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=8N1BxHgsoOw;License: Standard YouTube License, CC-BY