
Concept explainers
A
Figure 16.6. What is the structure of this diol?
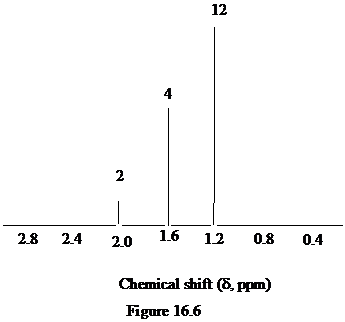
Interpretation:
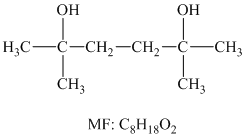
The structure for the diol having molecular formula
Concept introduction:
The vicinal diols on treatment with periodic acid
The oxygen atoms do not show any effect on the index of hydrogen deficiency.
The index of hydrogen deficiency (IHD) represents the presence of number of multiple bonds or a ring in a molecule which can be calculated from the following formula,
Here,
The
The chemical shift for the protons at carbon bonded to electron withdrawing group increases due to de-shielding effect.
Answer to Problem 36P
Solution: The structure for the diol having molecular formula
Explanation of Solution
As it is mentioned that the diol having molecular formula
As the oxygen atoms have no effect on index of hydrogen deficiency, the molecular formula of the given compound can be written as
Thus, the index of hydrogen deficiency in the given molecule can be calculated as follows:
This shows that the molecule does not have a ring or multiple bond. It is saturated compound.
The approximate chemical shift values from the given
a)
b)
c)
The arrangement of atoms in a molecule form the given chemical shift values are as follows:
The chemical shift at
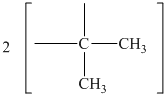
The peak at
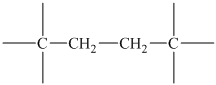
The peak at
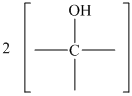
Hence, the structure of diol having molecular formula
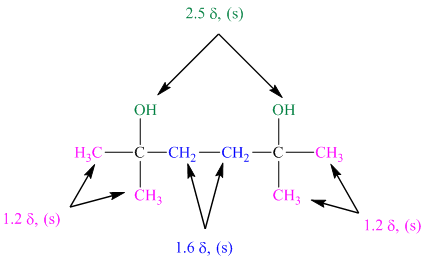
The structure of diol having molecular formula
Want to see more full solutions like this?
Chapter 16 Solutions
ORGANIC CHEMISTRY-PACKAGE >CUSTOM<
- Several diamines are building blocks for the synthesis of pharmaceuticals and agro-chemicals. Show how both 1,3-propanediamine and 1,4-butanediamine can be prepared from acrylonitrile.arrow_forwardAcetals are formed from the reaction of two alcohols with a carbonyl under acidic conditions. Acetal formation is faster with 1,2-ethanediol than with two methanol molecules. Choose the factor that explains the difference in reaction rates. A) The reaction with 1,2-ethanediol has a lower AH (enthalpy) of reaction. B) The reaction with 1,2-ethanediol has a higher AH (enthalpy) of reaction. C) The reaction with 1,2-ethanediol has a more favorable entropy of reaction.arrow_forwardWhen trichloroacetaldehyde is dissolved in water, almost all of it is converted to the hydrate. Chloral hydrate, the product of the reaction, is a sedative that can be lethal. A cocktail laced with it is known—in detective novels,at least—as a “Mickey Finn.” Explain why an aqueous solution of trichloroacetaldehyde is almost all hydrate.arrow_forward
- Provide structures A, B and Carrow_forwardIdentify A, B, and C, three intermediates in the synthesis of the pain reliever and anesthetic fentanyl.arrow_forwardWhich of the following reagents will convert salicylic acid into acetyl salicylic acid? HO HO, OCCH3 ethanol H2SO4 benzoic anhydride Methanol H2SO4 acetic anhydride thionyl chloridearrow_forward
- When trichloroacetaldehyde is dissolved in water, almost all of it is converted to the hydrate. Chloral hydrate, the product of the reaction, is a sedative that can be lethal. A cocktail laced with it is known—in detective novels, at least—as a “Mickey Finn.” Explain why an aqueous solution of trichloroacetaldehyde is almost all hydrate.arrow_forward4-Methylphenol is more acidic than ethanol (pKa 10.36 vs 16.0) , even though both contain an OH group and a methyl group. Draw the structures of the anions formed from loss of the alcoholic protons from both compounds. Use resonance to explain the difference in their respective acidities.arrow_forwardShow how to bring about each conversion in good yield.arrow_forward
- With reference to the structures of acetylsalicylic acid (aspirin) and acetaminophen (the active ingredient in Tylenol), explain why acetaminophen tablets can be stored in the medicine cabinet for years, but aspirin tablets slowly decompose over time.arrow_forwardWhat product is formed from the addition of NaOH to p-tertbutylphenol? Group of answer choices a compound with addition of an OH group to p-tertbutyl phenol the sodium salt of p-tertbutylphenol, sodium p-tertbutylphenoxide a protonated version of p-tertbutylphenol there is no reactionarrow_forwardDetermine and draw the structure of compound A onlyarrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning


