
Concept explainers
(a)
Interpretation:
Chemical equation for the preparation of given
Concept Introduction:
The name of the carboxylic acid itself implies that it is acidic. Addition of carboxylic acid to water results in ionization. Hydrogen ion transfer occurs from carboxylic acid to water and hydronium ion is formed. Carboxylate ion is also formed due to the loss of hydrogen ion from carboxylic acid.
Carboxylate ion is the negative ion which is formed when one or more acidic protons are lost from carboxylic acid. Similar to carboxylic acid it reacts with strong base to form carboxylic acid salt and water.
Carboxylic acid forms carboxylic acid salt by reacting with a strong base. The general reaction scheme for the formation of carboxylic acid salt is given as shown below,
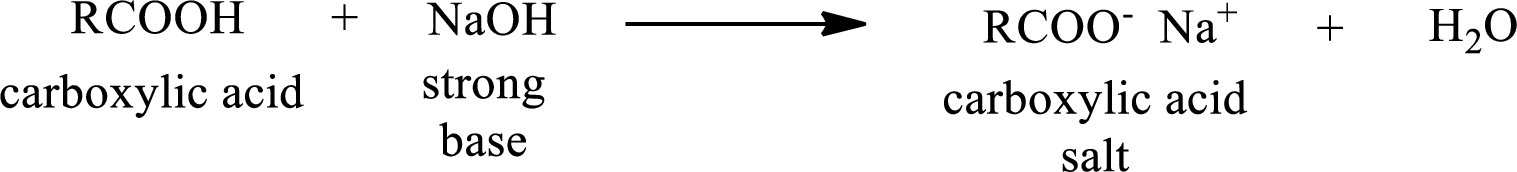
This is similar to the reaction of inorganic acids with base.
(b)
Interpretation:
Chemical equation for the preparation of given carboxylic acid salt using acid‑base neutralization reaction has to be given.
Concept Introduction:
The name of the carboxylic acid itself implies that it is acidic. Addition of carboxylic acid to water results in ionization. Hydrogen ion transfer occurs from carboxylic acid to water and hydronium ion is formed. Carboxylate ion is also formed due to the loss of hydrogen ion from carboxylic acid.
Carboxylate ion is the negative ion which is formed when one or more acidic protons are lost from carboxylic acid. Similar to carboxylic acid it reacts with strong base to form carboxylic acid salt and water.
Carboxylic acid forms carboxylic acid salt by reacting with a strong base. The general reaction scheme for the formation of carboxylic acid salt is given as shown below,
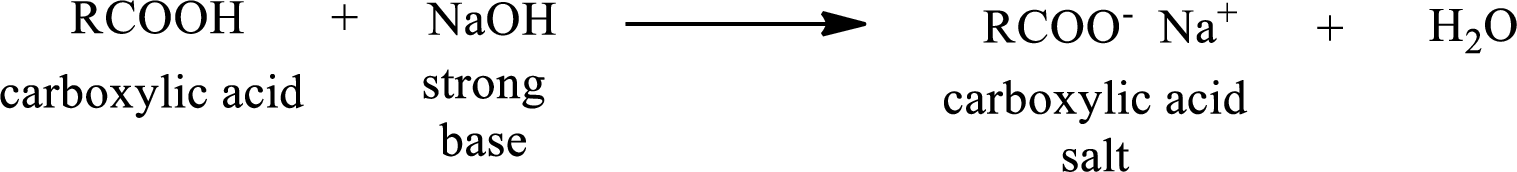
This is similar to the reaction of inorganic acids with base.
(c)
Interpretation:
Chemical equation for the preparation of given carboxylic acid salt using acid‑base neutralization reaction has to be given.
Concept Introduction:
The name of the carboxylic acid itself implies that it is acidic. Addition of carboxylic acid to water results in ionization. Hydrogen ion transfer occurs from carboxylic acid to water and hydronium ion is formed. Carboxylate ion is also formed due to the loss of hydrogen ion from carboxylic acid.
Carboxylate ion is the negative ion which is formed when one or more acidic protons are lost from carboxylic acid. Similar to carboxylic acid it reacts with strong base to form carboxylic acid salt and water.
Carboxylic acid forms carboxylic acid salt by reacting with a strong base. The general reaction scheme for the formation of carboxylic acid salt is given as shown below,
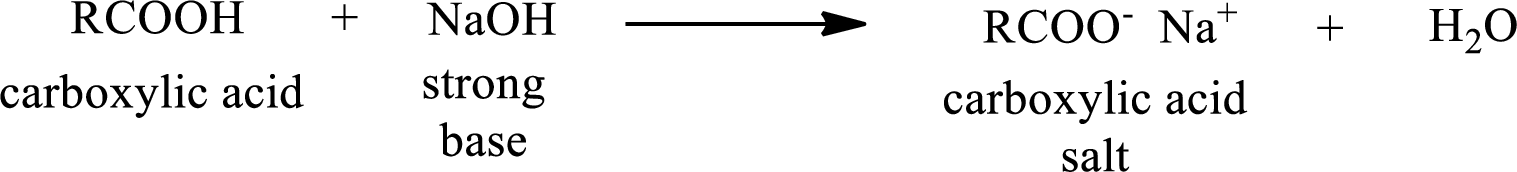
This is similar to the reaction of inorganic acids with base.
(d)
Interpretation:
Chemical equation for the preparation of given carboxylic acid salt using acid‑base neutralization reaction has to be given.
Concept Introduction:
The name of the carboxylic acid itself implies that it is acidic. Addition of carboxylic acid to water results in ionization. Hydrogen ion transfer occurs from carboxylic acid to water and hydronium ion is formed. Carboxylate ion is also formed due to the loss of hydrogen ion from carboxylic acid.
Carboxylate ion is the negative ion which is formed when one or more acidic protons are lost from carboxylic acid. Similar to carboxylic acid it reacts with strong base to form carboxylic acid salt and water.
Carboxylic acid forms carboxylic acid salt by reacting with a strong base. The general reaction scheme for the formation of carboxylic acid salt is given as shown below,
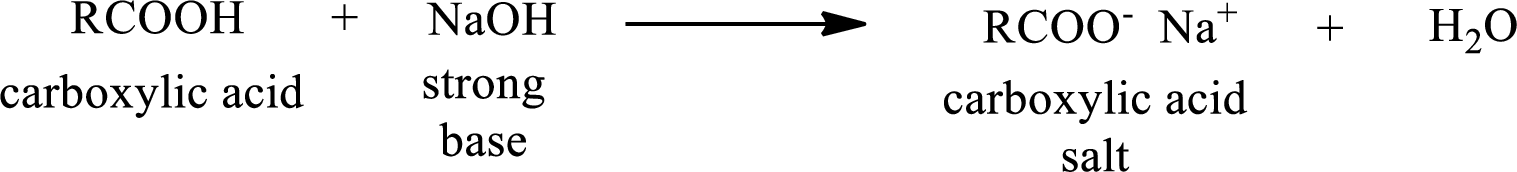
This is similar to the reaction of inorganic acids with base.
Trending nowThis is a popular solution!

Chapter 16 Solutions
Study Guide with Selected Solutions for Stoker's General, Organic, and Biological Chemistry, 7th
- What is the name of the following compound? SiMe3arrow_forwardK Draw the starting structure that would lead to the major product shown under the provided conditions. Drawing 1. NaNH2 2. PhCH2Br 4 57°F Sunny Q Searcharrow_forward7 Draw the starting alkyl bromide that would produce this alkyne under these conditions. F Drawing 1. NaNH2, A 2. H3O+ £ 4 Temps to rise Tomorrow Q Search H2arrow_forward
 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning



