
(a)
Interpretation:
Chemical equation for the conversion of given
Concept Introduction:
The name of the carboxylic acid itself implies that it is acidic. Addition of carboxylic acid to water results in ionization. Hydrogen ion transfer occurs from carboxylic acid to water and hydronium ion is formed. Carboxylate ion is also formed due to the loss of hydrogen ion from carboxylic acid.
Carboxylate ion is the negative ion which is formed when one or more acidic protons are lost from carboxylic acid. Similar to carboxylic acid it reacts with strong base to form carboxylic acid salt and water.
Carboxylic acid salts when treated with a strong acid produces carboxylic acid as the product.
Carboxylic acid forms carboxylic acid salt by reacting with a strong base. The general reaction scheme for the formation of carboxylic acid salt is given as shown below,
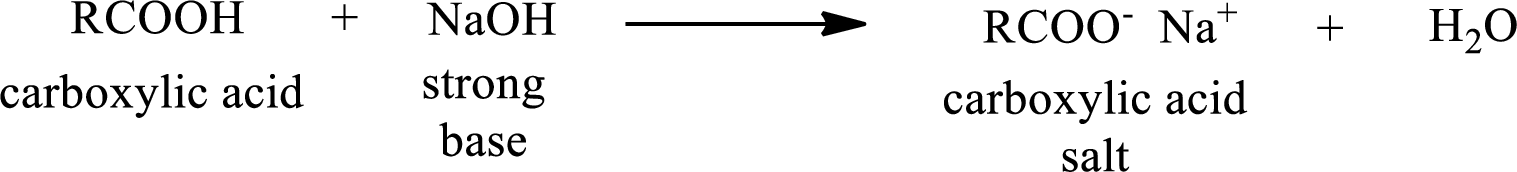
The reverse of the above reaction is conversion of carboxylic acid salt to carboxylic acid. This is accomplished by using strong acid. The scheme is shown below,
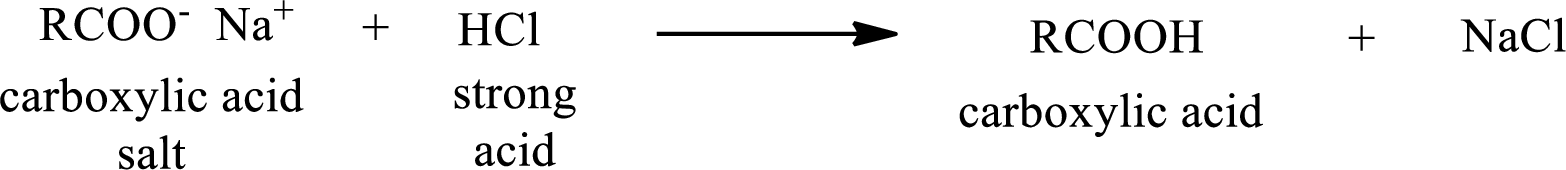
From the above chemical equation it is found that the carboxylic acid salt reacts with strong acid to form carboxylic acid.
(a)
Explanation of Solution
Given carboxylic acid salt is sodium butanoate. The structure of sodium butanoate can be given as shown below,
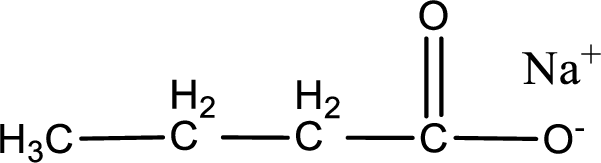
Carboxylic acid sat is converted to carboxylic acid by reaction with strong acid. In the problem statement it is given that the strong acid is
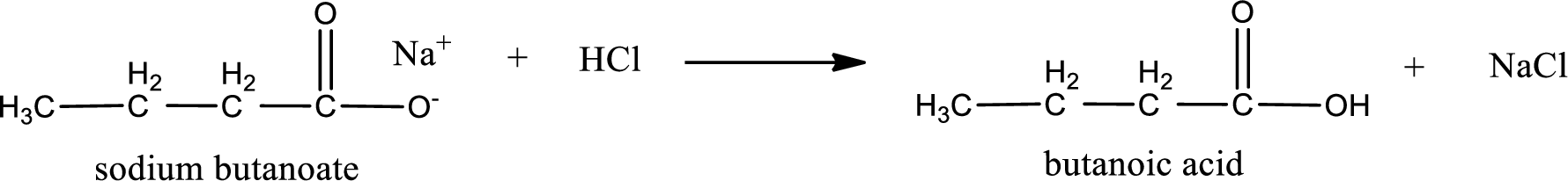
Chemical equation for the conversion of given carboxylic acid salt to carboxylic acid using hydrochloric acid is written.
(b)
Interpretation:
Chemical equation for the conversion of given carboxylic acid salt to its parent carboxylic acid using
Concept Introduction:
The name of the carboxylic acid itself implies that it is acidic. Addition of carboxylic acid to water results in ionization. Hydrogen ion transfer occurs from carboxylic acid to water and hydronium ion is formed. Carboxylate ion is also formed due to the loss of hydrogen ion from carboxylic acid.
Carboxylate ion is the negative ion which is formed when one or more acidic protons are lost from carboxylic acid. Similar to carboxylic acid it reacts with strong base to form carboxylic acid salt and water.
Carboxylic acid salts when treated with a strong acid produces carboxylic acid as the product.
Carboxylic acid forms carboxylic acid salt by reacting with a strong base. The general reaction scheme for the formation of carboxylic acid salt is given as shown below,
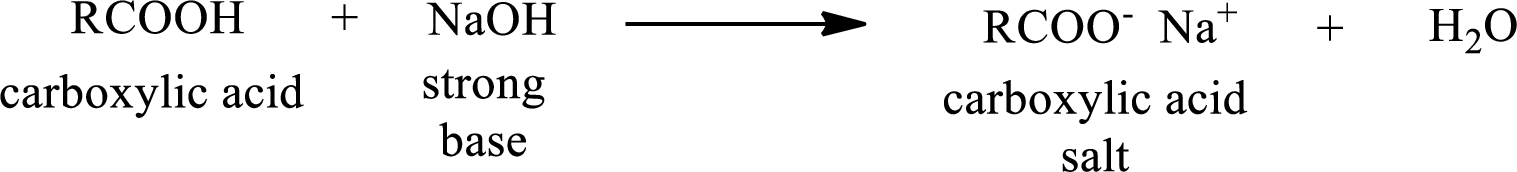
The reverse of the above reaction is conversion of carboxylic acid salt to carboxylic acid. This is accomplished by using strong acid. The scheme is shown below,
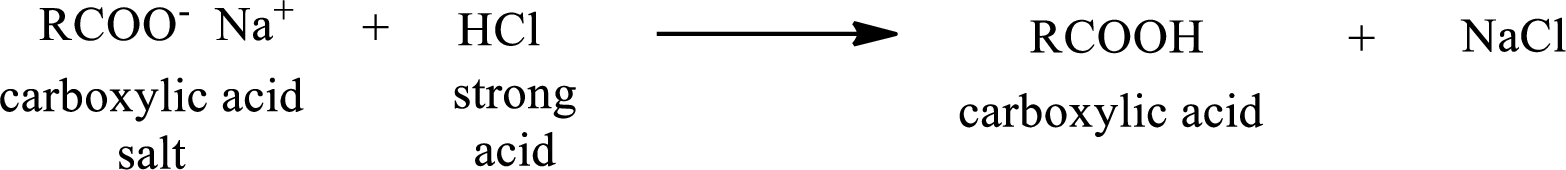
From the above chemical equation it is found that the carboxylic acid salt reacts with strong acid to form carboxylic acid.
(b)
Explanation of Solution
Given carboxylic acid salt is potassium oxalate. The structure of potassium oxalate can be given as shown below,
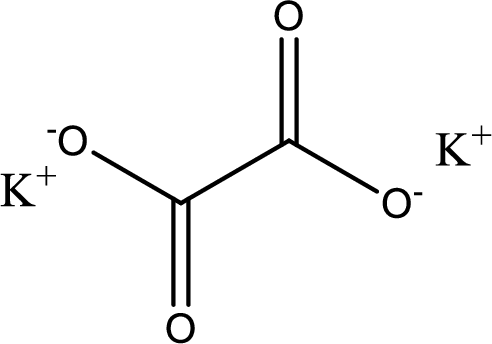
Carboxylic acid sat is converted to carboxylic acid by reaction with strong acid. In the problem statement it is given that the strong acid is
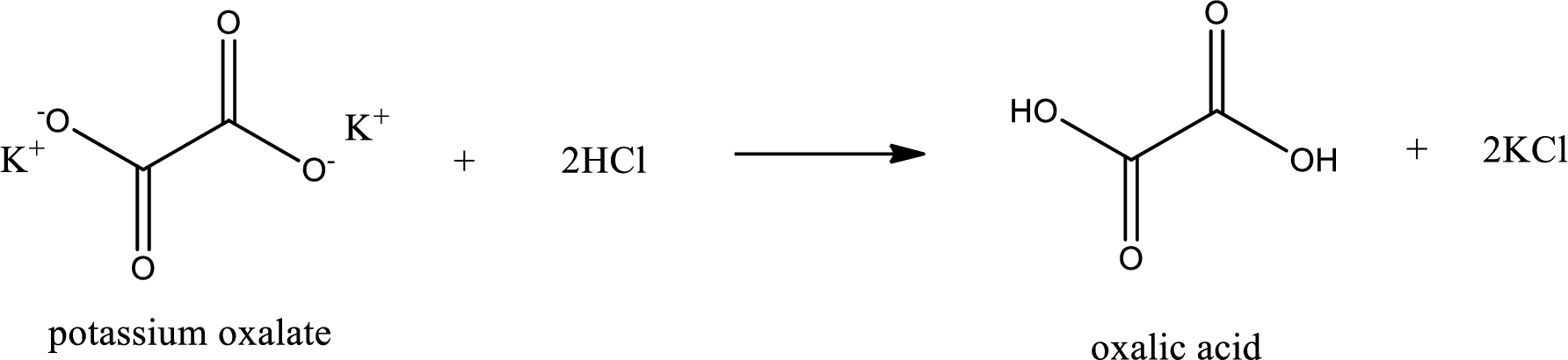
Chemical equation for the conversion of given carboxylic acid salt to carboxylic acid using hydrochloric acid is written.
(c)
Interpretation:
Chemical equation for the conversion of given carboxylic acid salt to its parent carboxylic acid using
Concept Introduction:
The name of the carboxylic acid itself implies that it is acidic. Addition of carboxylic acid to water results in ionization. Hydrogen ion transfer occurs from carboxylic acid to water and hydronium ion is formed. Carboxylate ion is also formed due to the loss of hydrogen ion from carboxylic acid.
Carboxylate ion is the negative ion which is formed when one or more acidic protons are lost from carboxylic acid. Similar to carboxylic acid it reacts with strong base to form carboxylic acid salt and water.
Carboxylic acid salts when treated with a strong acid produces carboxylic acid as the product.
Carboxylic acid forms carboxylic acid salt by reacting with a strong base. The general reaction scheme for the formation of carboxylic acid salt is given as shown below,
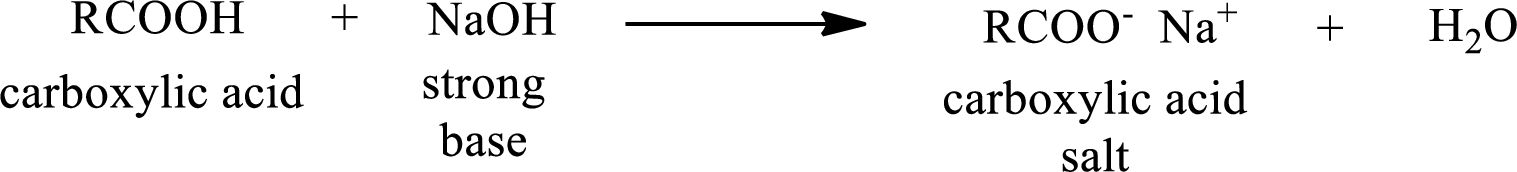
The reverse of the above reaction is conversion of carboxylic acid salt to carboxylic acid. This is accomplished by using strong acid. The scheme is shown below,
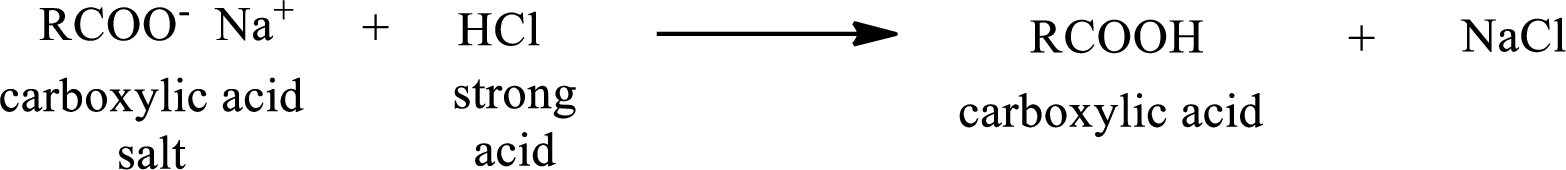
From the above chemical equation it is found that the carboxylic acid salt reacts with strong acid to form carboxylic acid.
(c)
Explanation of Solution
Given carboxylic acid salt is calcium malonate. The structure of calcium malonate can be given as shown below,
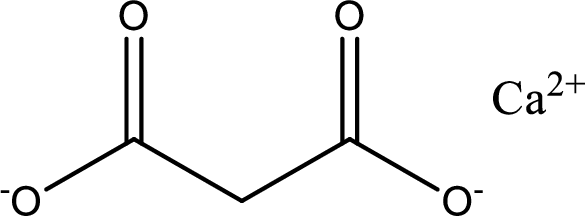
Carboxylic acid sat is converted to carboxylic acid by reaction with strong acid. In the problem statement it is given that the strong acid is
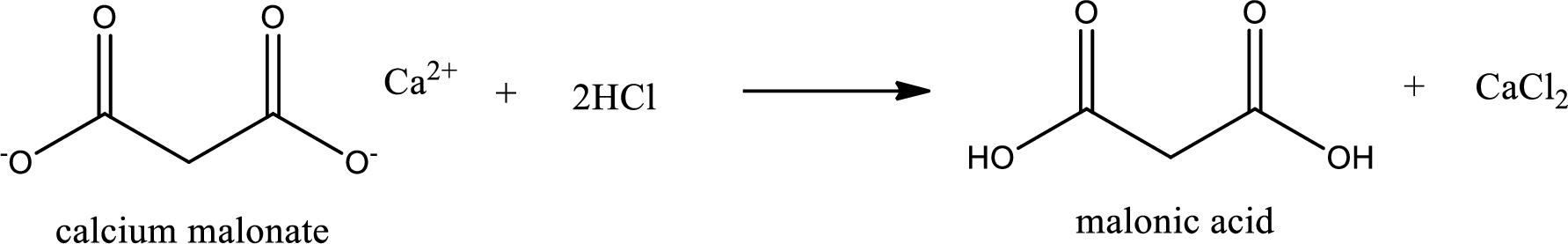
Chemical equation for the conversion of given carboxylic acid salt to carboxylic acid using hydrochloric acid is written.
(d)
Interpretation:
Chemical equation for the conversion of given carboxylic acid salt to its parent carboxylic acid using
Concept Introduction:
The name of the carboxylic acid itself implies that it is acidic. Addition of carboxylic acid to water results in ionization. Hydrogen ion transfer occurs from carboxylic acid to water and hydronium ion is formed. Carboxylate ion is also formed due to the loss of hydrogen ion from carboxylic acid.
Carboxylate ion is the negative ion which is formed when one or more acidic protons are lost from carboxylic acid. Similar to carboxylic acid it reacts with strong base to form carboxylic acid salt and water.
Carboxylic acid salts when treated with a strong acid produces carboxylic acid as the product.
Carboxylic acid forms carboxylic acid salt by reacting with a strong base. The general reaction scheme for the formation of carboxylic acid salt is given as shown below,
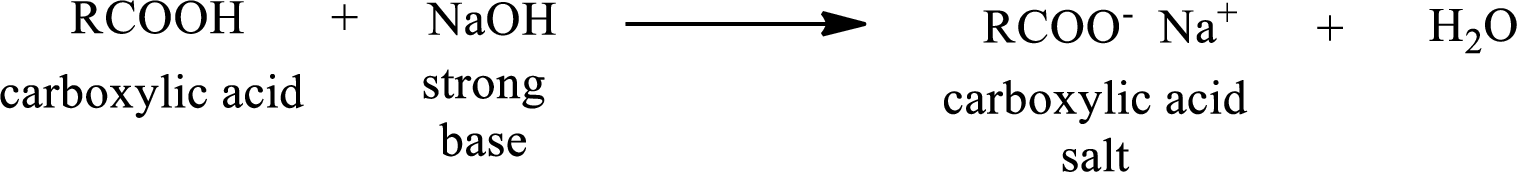
The reverse of the above reaction is conversion of carboxylic acid salt to carboxylic acid. This is accomplished by using strong acid. The scheme is shown below,
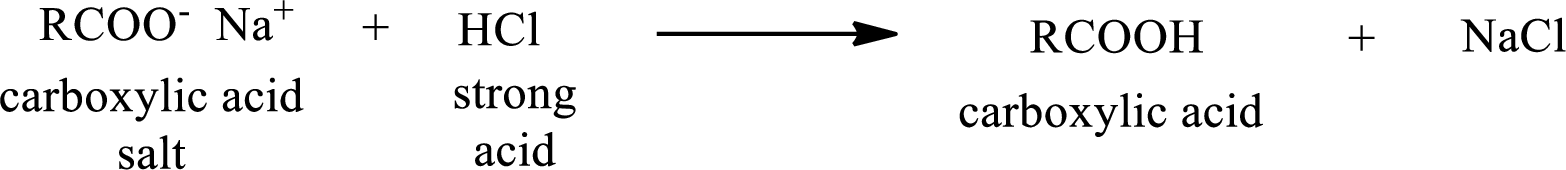
From the above chemical equation it is found that the carboxylic acid salt reacts with strong acid to form carboxylic acid.
(d)
Explanation of Solution
Given carboxylic acid salt is sodium benzoate. The structure of sodium benzoate can be given as shown below,
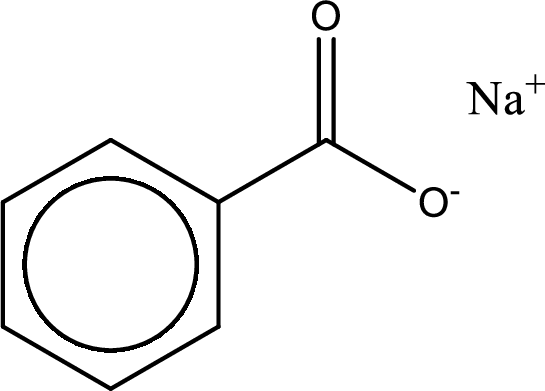
Carboxylic acid sat is converted to carboxylic acid by reaction with strong acid. In the problem statement it is given that the strong acid is
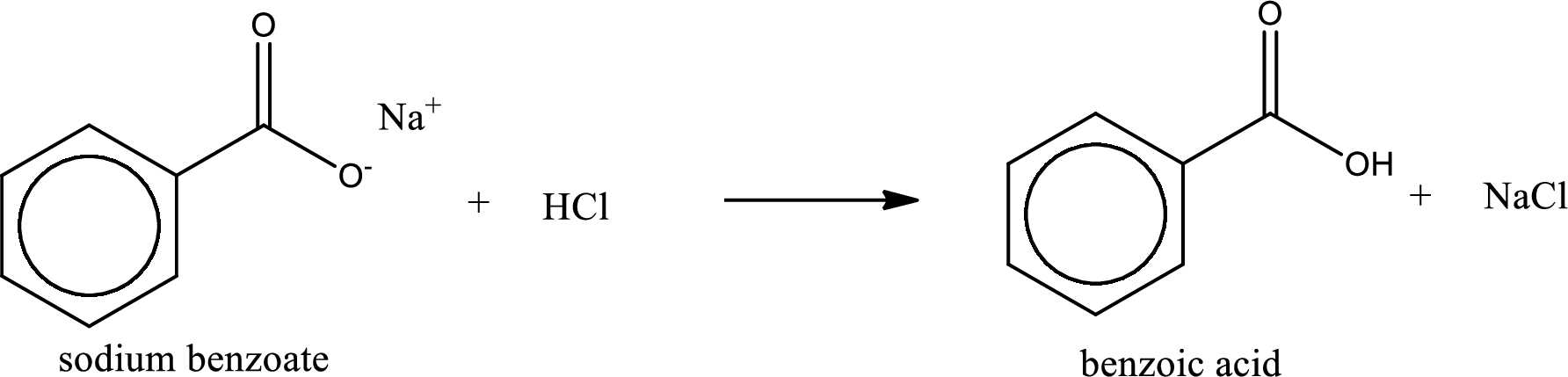
Chemical equation for the conversion of given carboxylic acid salt to carboxylic acid using hydrochloric acid is written.
Want to see more full solutions like this?
Chapter 16 Solutions
Study Guide with Selected Solutions for Stoker's General, Organic, and Biological Chemistry, 7th
- If I have 10 data points for variables x and y, when I represent y versus x I obtain a line with the equation y = mx + b. Is the slope m equal to dy/dx?arrow_forwardThe data for the potential difference of a battery and its temperature are given in the table. Calculate the entropy change in J mol-1 K-1 (indicate the formulas used).Data: F = 96485 C mol-1arrow_forwardIn a cell, the change in entropy (AS) can be calculated from the slope of the E° vs 1/T graph. The slope is equal to -AS/R, where R is the gas constant. Is this correct?arrow_forward
- Using the Arrhenius equation, it is possible to establish the relationship between the rate constant (k) of a chemical reaction and the temperature (T), in Kelvin (K), the universal gas constant (R), the pre-exponential factor (A) and the activation energy (Ea). This equation is widely applied in studies of chemical kinetics, and is also widely used to determine the activation energy of reactions. In this context, the following graph shows the variation of the rate constant with the inverse of the absolute temperature, for a given chemical reaction that obeys the Arrhenius equation. Based on the analysis of this graph and the concepts acquired about the kinetics of chemical reactions, analyze the following statements: I. The activation energy (Ea) varies with the temperature of the system. II. The activation energy (Ea) varies with the concentration of the reactants. III. The rate constant (K) varies proportionally with temperature. IV. The value of the…arrow_forwardIn an electrolytic cell, indicate the formula that relates E0 to the temperature T.arrow_forward-- 14:33 A Candidate Identification docs.google.com 11. Compound A can transform into compound B through an organic reaction. From the structures below, mark the correct one: HO A تھے۔ די HO B ○ A) Compounds A and B are isomers. B) Both have the same number of chiral carbons. C) Compound A underwent an addition reaction of Cl2 and H2O to form compound B. D) Compound A underwent a substitution reaction forming the intermediate chlorohydrin to obtain compound B. E) Compound A underwent an addition reaction of Cl2 forming the chloronium ion and then added methanol to obtain compound B. 60arrow_forward
- -- 14:40 A Candidate Identification docs.google.com 13. The compound 1-bromo-hex-2-ene reacts with methanol to form two products. About this reaction, mark the correct statement: OCH3 CH3OH Br OCH3 + + HBr A B A) The two products formed will have the same percentage of formation. B) Product B will be formed by SN1 substitution reaction with the formation of an allylic carbocation. C) Product A will be formed by SN1 substitution reaction with the formation of a more stable carbocation than product B. D) Product A will be formed by an SN2 substitution reaction occurring in two stages, the first with slow kinetics and the second with fast kinetics. E) The two compounds were obtained by addition reaction, with compound B having the highest percentage of formation. 57arrow_forward-- ☑ 14:30 A Candidate Identification docs.google.com 10. Amoxicillin (figure X) is one of the most widely used antibiotics in the penicillin family. The discovery and synthesis of these antibiotics in the 20th century made the treatment of infections that were previously fatal routine. About amoxicillin, mark the correct one: HO NH2 H S -N. HO Figura X. Amoxicilina A) It has the organic functions amide, ester, phenol and amine. B) It has four chiral carbons and 8 stereoisomers. C) The substitution of the aromatic ring is of the ortho-meta type. D) If amoxicillin reacts with an alcohol it can form an ester. E) The structure has two tertiary amides. 62arrow_forwardThe environmental police of a Brazilian state received a report of contamination of a river by inorganic arsenic, due to the excessive use of pesticides on a plantation on the riverbanks. Arsenic (As) is extremely toxic in its many forms and oxidation states. In nature, especially in groundwater, it is found in the form of arsenate (AsO ₄ ³ ⁻ ), which can be electrochemically reduced to As ⁰ and collected at the cathode of a coulometric cell. In this case, Potentiostatic Coulometry (at 25°C) was performed in an alkaline medium (pH = 7.5 throughout the analysis) to quantify the species. What potential (E) should have been selected/applied to perform the analysis, considering that this is an exhaustive electrolysis technique (until 99.99% of all AsO ₄ ³ ⁻ has been reduced to As ⁰ at the electrode, or n( final) = 0.01% n( initial )) and that the concentration of AsO ₄ ³ ⁻ found in the initial sample was 0.15 mmol/L ? Data: AsO ₄ 3 ⁻ (aq) + 2 H ₂ O ( l ) + 2 e ⁻ → A s O ₂ ⁻ ( a…arrow_forward
- -- 14:17 15. Water-soluble proteins are denatured when there is a change in the pH of the environment in which they are found. This occurs due to the protonation and deprotonation of functional groups present in their structure. Choose the option that indicates the chemical bonds modified by pH in the protein represented in the following figure. E CH2 C-OH CH2 H₂C H₁C CH CH3 CH3 CH CH₂-S-S-CH₂- 910 H B -CH2-CH2-CH2-CH₂-NH3* −0—C—CH₂- ○ A) A, C e D. • В) Вес ○ C) DeE ○ D) B, De E ○ E) A, B e C 68arrow_forwardSuppose sodium sulfate has been gradually added to 100 mL of a solution containing calcium ions and strontium ions, both at 0.15 mol/L. Indicate the alternative that presents the percentage of strontium ions that will have precipitated when the calcium sulfate begins to precipitate. Data: Kps of calcium sulfate: 2.4x10 ⁻ ⁵; Kps of strontium sulfate: 3.2x10 ⁻ ⁷ A) 20,2 % B) 36,6 % C) 62,9 % D) 87,5 % E) 98.7%arrow_forward14:43 A Candidate Identification docs.google.com 14. The following diagrams represent hypothetical membrane structures with their components numbered from 1 to 6. Based on the figures and your knowledge of biological membranes, select the correct alternative. | 3 5 || 人 2 500000 6 A) Structures 1, 3, 5, 2 and 4 are present in a constantly fluid arrangement that allows the selectivity of the movement ○ of molecules. Structure 4, present integrally or peripherally, is responsible for this selection, while the quantity of 6 regulates the fluidity. B) The membranes isolate the cell from the environment, but allow the passage of water-soluble molecules thanks to the presence of 2 and 3. The membrane in scheme is more fluid than that in 55arrow_forward
 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





