
(a)
Interpretation:
The structure of the product formed in the given reaction is to be shown.
Concept Introduction:
In the Friedel-craft alkylation reaction alkyl chloride and benzene reacts to form alkylated benzene compound. The Lewis acid
Answer to Problem 16.61AP
The structure of product formed in the given reaction is shown below.
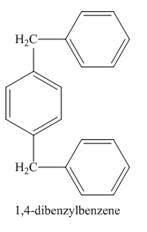
Explanation of Solution
The given incomplete reaction is shown below.
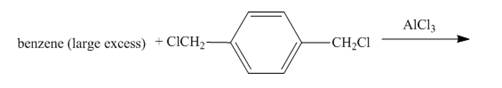
Figure 1
The benzene ring in the presence of
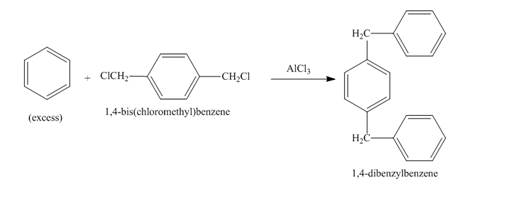
Figure 2
The structure of compound formed in the given reaction is shown in Figure 2.
(b)
Interpretation:
The structure of the product formed in the given reaction is to be shown.
Concept Introduction:
In the Friedel craft alkylation reaction alkyl chloride and benzene reacts to form alkylated benzene compound. The Lewis acid
Answer to Problem 16.61AP
The structure of product formed in the given reaction is shown below.
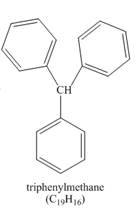
Explanation of Solution
The incomplete reaction is shown below.
The benzene reacts with chloroform to form dichloromethyl benzene. Aluminium chloride acts as catalyst in this reaction. As benzene is present in excess amount, it will again react with the dichloromethyl benzene to form triphenyl methane. The complete reaction is shown below.
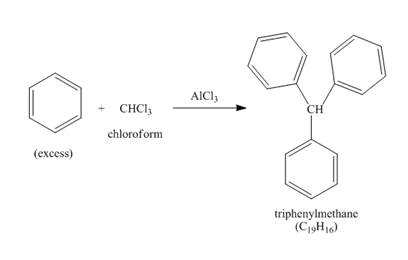
Figure 3
The structure of compound formed in the given reaction is shown in Figure 3.
(c)
Interpretation:
The structure of the product formed in the given reaction is to be shown.
Concept Introduction:
In the Friedel craft alkylation reaction alkyl chloride and benzene reacts to form alkylated benzene compound. The Lewis acid
Answer to Problem 16.61AP
The structure of product formed in the given reaction is shown below.
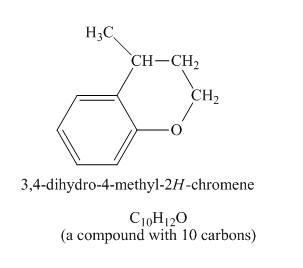
Explanation of Solution
The incomplete reaction is shown below.
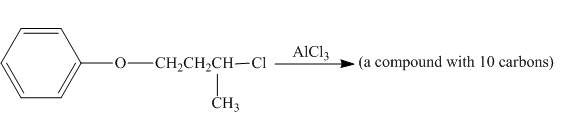
Figure 4
The given compound undergoes Friedel craft alkylation reaction to form the cyclic compound. The product formed in the given reaction is
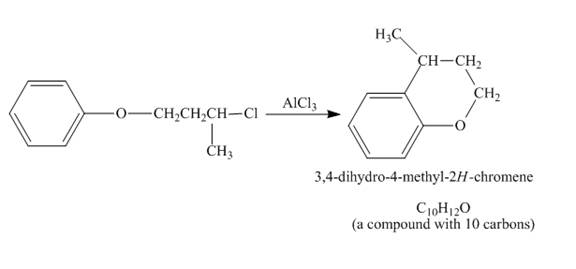
Figure 5
The structure of compound formed in the given reaction is shown in Figure 5.
(d)
Interpretation:
The structure of the product formed in the given reaction is to be shown.
Concept Introduction:
Reaction of benzene with acyl chloride in presence of Lewis acid like aluminium tricloride takes place to form acylated benzene. This reaction is known as Friedel-crafts acylation reaction. The electrophile in this reaction is a carbocation, known as acylium ion. This ion is formed when acid chloride reacts with Lewis acid.
Answer to Problem 16.61AP
The structure of product formed in the given reaction is shown below.
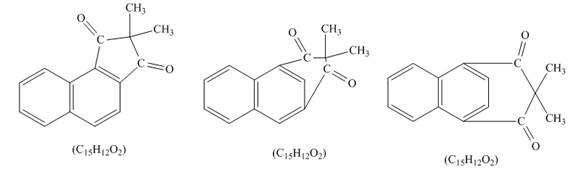
Explanation of Solution
The given incomplete reaction is shown below.
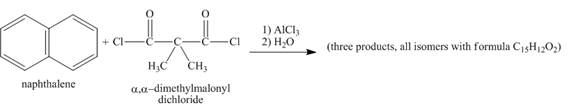
Figure 6
Naphthalene reacts with
Figure 7
The structure of the product formed in the given reaction is shown in Figure 7.
(e)
Interpretation:
The structure of the product formed in the given reaction is to be shown.
Concept Introduction:
The substituted benzene ring when undergoes electrophilic substitution reaction, then it will form ortho, meta or para compounds. If the substituent present on the ring is directing the electrophile at ortho-para position, then it an ortho-para directing group. If the already present substituents direct the incoming electrophile to meta position, then it is a meta directing group. Hydroxyl group is ortho-para directing group.
Answer to Problem 16.61AP
The structure of product formed in the given reaction is shown below.
Explanation of Solution
The incomplete reaction is shown below.
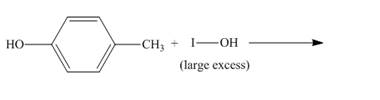
Figure 8
The compound
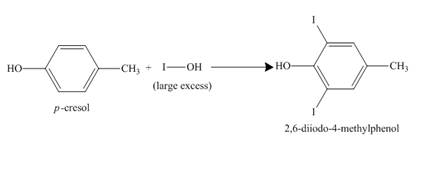
Figure 9
The structure of the product formed in the given reaction is shown in Figure 9.
(f)
Interpretation:
The structure of the product formed in the given reaction is to be shown.
Concept Introduction:
The substituted benzene ring when further undergoes electrophilic substitution reaction, and then it will form ortho, meta or para compounds. If the substituent present on the ring is directing the electrophile at ortho-para position, then it an ortho-para directing group. If the already present substituent directs the incoming electrophile to meta position then it is a meta directing group. Cyclohexyl group is ortho-para directing group.
Answer to Problem 16.61AP
The structure of product formed in the given reaction is shown below.
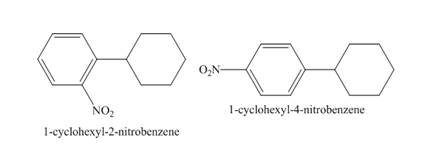
Explanation of Solution
The given incomplete reaction is shown below.
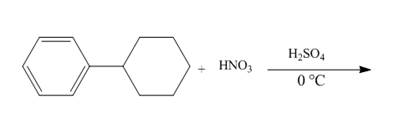
Figure 10
The compound
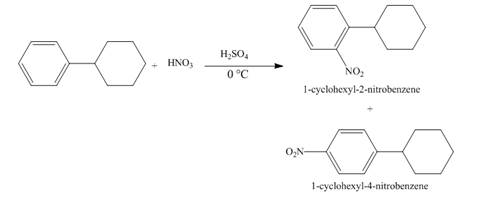
Figure 11
The structure of the product formed in the given reaction is shown in Figure 11.
(g)
Interpretation:
The structure of the product formed in the given reaction is to be shown.
Concept Introduction:
Reaction of benzene with acyl chloride in presence of Lewis acid like aluminium tricloride to form acylated benzene is known as Friedel-crafts acylation reaction. The electrophile in the reaction is a carbocation, known as acylium ion. This ion is formed when acid chloride reacts with Lewis acid.
Answer to Problem 16.61AP
The structure of product formed in the given reaction is shown below.
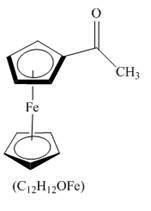
Explanation of Solution
The given incomplete reaction is shown below.
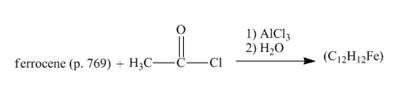
Figure 12
The compound ferrocene reacts with acyl chloride to form acylated ferrocene ring. The acetyl group is an electrophile. The electrophilic substitution reaction of ferrocene takes place to form acylated ferrocene. The
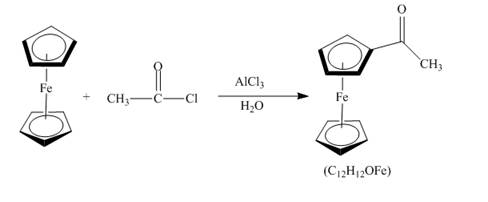
Figure 13
The structure of the product formed in the given reaction is shown in Figure 13.
(h)
Interpretation:
The structure of the product formed in the given reaction is to be shown.
Concept Introduction:
Nitration reaction takes place in the presence of nitric acid. It is an electrophilic substitution reaction. Nitronium ion is formed as electrophile in nitration reaction. The methoxy group is an activating group, it is ortho-para directing group. The nitro group is strongly deactivating group and it is meta directing group.
Answer to Problem 16.61AP
The structure of product formed in the given reaction is shown below.
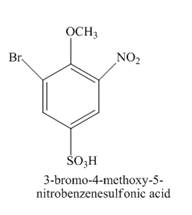
Explanation of Solution
The given incomplete reaction is shown below.
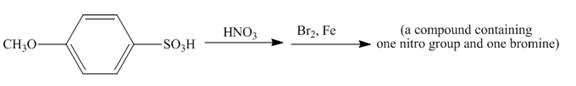
Figure 14
The reaction of
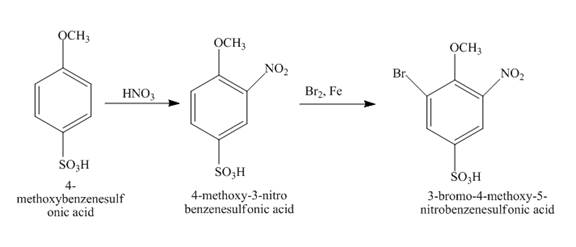
Figure 15
The structure of the product formed in the given reaction is shown in Figure 15.
Want to see more full solutions like this?
Chapter 16 Solutions
Organic Chemistry
- Identify any polar covalent bonds in epichlorohydrin with S+ and 8- symbols in the appropriate locations. Choose the correct answer below. Η H's+ 6Η Η Η Η Η Ηδ Η Ο Ο HH +Η Η +Η Η Η -8+ CIarrow_forwardH H:O::::H H H HH H::O:D:D:H HH HH H:O:D:D:H .. HH H:O:D:D:H H H Select the correct Lewis dot structure for the following compound: CH3CH2OHarrow_forwardRank the following compounds in order of decreasing boiling point. ннннн -С-С-Н . н-с- ННННН H ΗΤΗ НННН TTTĪ н-с-с-с-с-о-н НННН НН C' Н н-с-с-с-с-н НН || Ш НННН H-C-C-C-C-N-H ННННН IVarrow_forward
- Rank the following compounds in order of decreasing dipole moment. |>||>||| ||>|||>| |>|||>|| |||>||>| O ||>>||| H F H F H c=c || H c=c F F IIIarrow_forwardchoose the description that best describes the geometry for the following charged species ch3-arrow_forwardWhy isn't the ketone in this compound converted to an acetal or hemiacetal by the alcohol and acid?arrow_forward
- What is the approximate bond angle around the nitrogen atom? HNH H Harrow_forwardOH 1. NaOCH2CH3 Q 2. CH3CH2Br (1 equiv) H3O+ Select to Draw 1. NaOCH2 CH3 2. CH3Br (1 equiv) heat Select to Edit Select to Drawarrow_forwardComplete and balance the following half-reaction in acidic solution. Be sure to include the proper phases for all species within the reaction. S₂O₃²⁻(aq) → S₄O₆²⁻(aq)arrow_forward
- Q Select to Edit NH3 (CH3)2CHCI (1 equiv) AICI 3 Select to Draw cat. H2SO4 SO3 (1 equiv) HO SOCl2 pyridine Select to Edit >arrow_forwardComplete and balance the following half-reaction in basic solution. Be sure to include the proper phases for all species within the reaction. Zn(s) → Zn(OH)₄²⁻(aq)arrow_forwardb. ὋΗ CH3CH2OH H2SO4arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

