
(a)
Interpretation:
The structure of the product formed in the given reaction is to be shown.
Concept Introduction:
In the Friedel-craft alkylation reaction alkyl chloride and benzene reacts to form alkylated benzene compound. The Lewis acid
Answer to Problem 16.61AP
The structure of product formed in the given reaction is shown below.
Explanation of Solution
The given incomplete reaction is shown below.
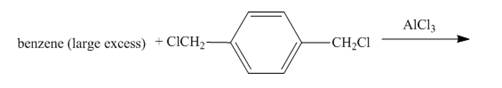
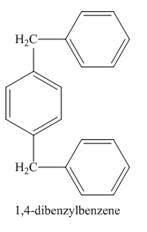
Figure 1
The benzene ring in the presence of
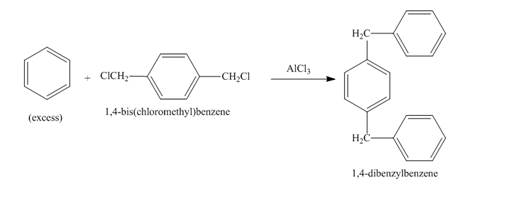
Figure 2
The structure of compound formed in the given reaction is shown in Figure 2.
(b)
Interpretation:
The structure of the product formed in the given reaction is to be shown.
Concept Introduction:
In the Friedel craft alkylation reaction alkyl chloride and benzene reacts to form alkylated benzene compound. The Lewis acid
Answer to Problem 16.61AP
The structure of product formed in the given reaction is shown below.
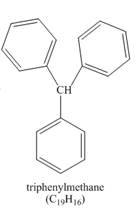
Explanation of Solution
The incomplete reaction is shown below.
The benzene reacts with chloroform to form dichloromethyl benzene. Aluminium chloride acts as catalyst in this reaction. As benzene is present in excess amount, it will again react with the dichloromethyl benzene to form triphenyl methane. The complete reaction is shown below.
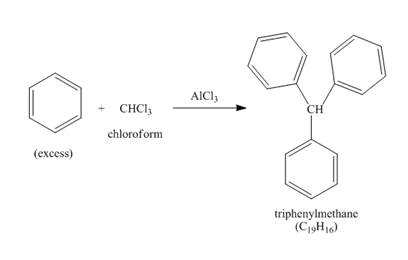
Figure 3
The structure of compound formed in the given reaction is shown in Figure 3.
(c)
Interpretation:
The structure of the product formed in the given reaction is to be shown.
Concept Introduction:
In the Friedel craft alkylation reaction alkyl chloride and benzene reacts to form alkylated benzene compound. The Lewis acid
Answer to Problem 16.61AP
The structure of product formed in the given reaction is shown below.
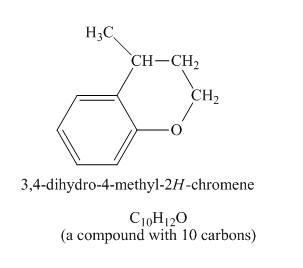
Explanation of Solution
The incomplete reaction is shown below.
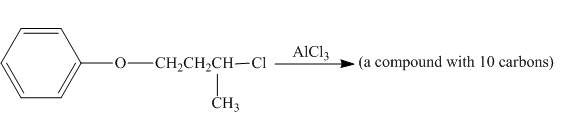
Figure 4
The given compound undergoes Friedel craft alkylation reaction to form the cyclic compound. The product formed in the given reaction is
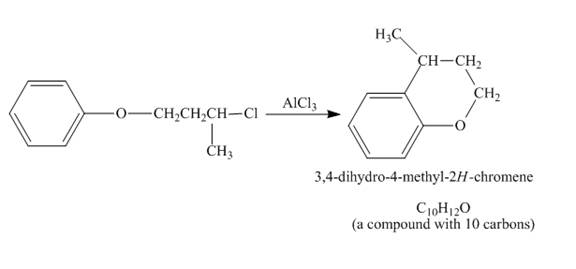
Figure 5
The structure of compound formed in the given reaction is shown in Figure 5.
(d)
Interpretation:
The structure of the product formed in the given reaction is to be shown.
Concept Introduction:
Reaction of benzene with acyl chloride in presence of Lewis acid like aluminium tricloride takes place to form acylated benzene. This reaction is known as Friedel-crafts acylation reaction. The electrophile in this reaction is a carbocation, known as acylium ion. This ion is formed when acid chloride reacts with Lewis acid.
Answer to Problem 16.61AP
The structure of product formed in the given reaction is shown below.
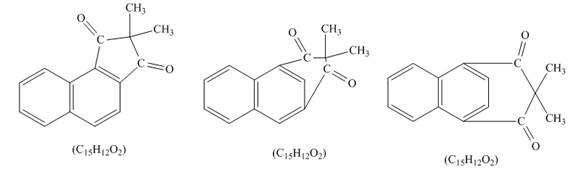
Explanation of Solution
The given incomplete reaction is shown below.
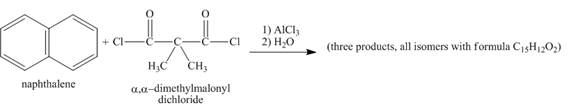
Figure 6
Naphthalene reacts with
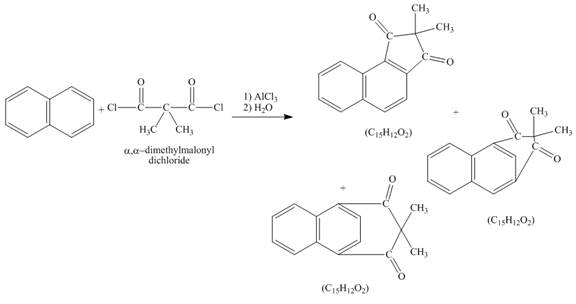
Figure 7
The structure of the product formed in the given reaction is shown in Figure 7.
(e)
Interpretation:
The structure of the product formed in the given reaction is to be shown.
Concept Introduction:
The substituted benzene ring when undergoes electrophilic substitution reaction, then it will form ortho, meta or para compounds. If the substituent present on the ring is directing the electrophile at ortho-para position, then it an ortho-para directing group. If the already present substituents direct the incoming electrophile to meta position, then it is a meta directing group. Hydroxyl group is ortho-para directing group.
Answer to Problem 16.61AP
The structure of product formed in the given reaction is shown below.
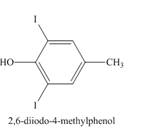
Explanation of Solution
The incomplete reaction is shown below.
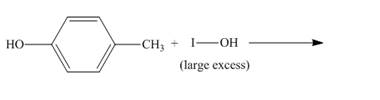
Figure 8
The compound
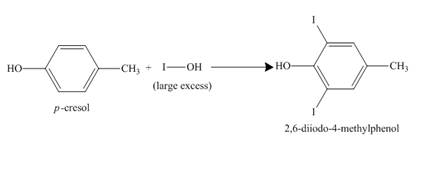
Figure 9
The structure of the product formed in the given reaction is shown in Figure 9.
(f)
Interpretation:
The structure of the product formed in the given reaction is to be shown.
Concept Introduction:
The substituted benzene ring when further undergoes electrophilic substitution reaction, and then it will form ortho, meta or para compounds. If the substituent present on the ring is directing the electrophile at ortho-para position, then it an ortho-para directing group. If the already present substituent directs the incoming electrophile to meta position then it is a meta directing group. Cyclohexyl group is ortho-para directing group.
Answer to Problem 16.61AP
The structure of product formed in the given reaction is shown below.
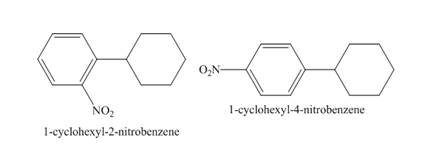
Explanation of Solution
The given incomplete reaction is shown below.
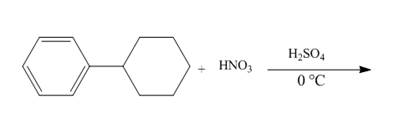
Figure 10
The compound
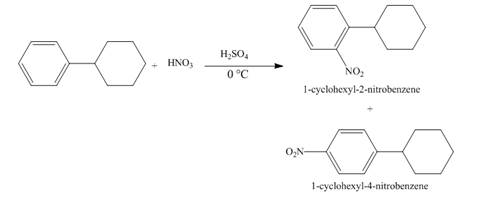
Figure 11
The structure of the product formed in the given reaction is shown in Figure 11.
(g)
Interpretation:
The structure of the product formed in the given reaction is to be shown.
Concept Introduction:
Reaction of benzene with acyl chloride in presence of Lewis acid like aluminium tricloride to form acylated benzene is known as Friedel-crafts acylation reaction. The electrophile in the reaction is a carbocation, known as acylium ion. This ion is formed when acid chloride reacts with Lewis acid.
Answer to Problem 16.61AP
The structure of product formed in the given reaction is shown below.
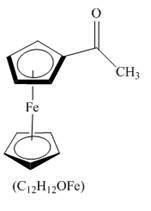
Explanation of Solution
The given incomplete reaction is shown below.
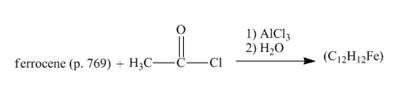
Figure 12
The compound ferrocene reacts with acyl chloride to form acylated ferrocene ring. The acetyl group is an electrophile. The electrophilic substitution reaction of ferrocene takes place to form acylated ferrocene. The
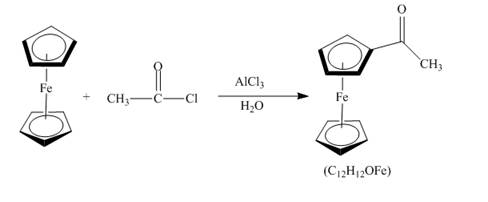
Figure 13
The structure of the product formed in the given reaction is shown in Figure 13.
(h)
Interpretation:
The structure of the product formed in the given reaction is to be shown.
Concept Introduction:
Nitration reaction takes place in the presence of nitric acid. It is an electrophilic substitution reaction. Nitronium ion is formed as electrophile in nitration reaction. The methoxy group is an activating group, it is ortho-para directing group. The nitro group is strongly deactivating group and it is meta directing group.
Answer to Problem 16.61AP
The structure of product formed in the given reaction is shown below.
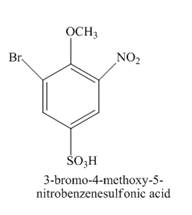
Explanation of Solution
The given incomplete reaction is shown below.
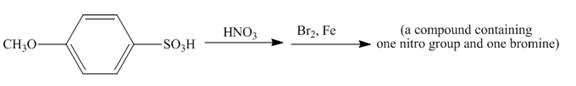
Figure 14
The reaction of
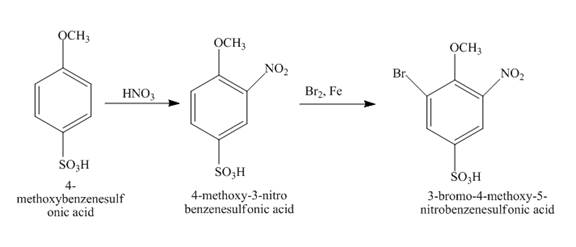
Figure 15
The structure of the product formed in the given reaction is shown in Figure 15.
Want to see more full solutions like this?
Chapter 16 Solutions
EBK ORGANIC CHEMISTRY
- CHEM 310 Quiz 8 Organic Chemistry II Due: Tuesday, April 25th, at 11:59 pm. This quiz is open textbook / open notes - but you must work alone. You cannot use the internet or the solutions manual for the book. Scan in your work and record an explanation of your mechanism. You may record this any way that you like. One way would be to start an individual Zoom meeting, start recording, "share your screen" and then talk through the problem. This will be converted to an .mp4 file that you can upload into Canvas using the "record/upload media" feature. Pyridine, benzoic acid and benzene are dissolved in ethyl acetate. Design and provide a plan / flow chart for separating and isolating each of these components. Pyridine and benzene are liquids at room temperature. Benzoic acid is a solid. You have ethyl acetate, 2M NaOH, 2M HCI and anhydrous MgSO4 available, as well as all the glassware and equipment that you used in the organic lab this year. Provide accurate acid/base reactions for any…arrow_forwardCan anyone help me solve this step by step. Thank you in advaarrow_forwardPlease draw the mechanism for this Friedel-crafts acylation reaction using arrowsarrow_forward
- Draw the Fischer projection of D-fructose. Click and drag to start drawing a structure. Skip Part Check AP 14 tv SC F1 F2 80 F3 a F4 ! 2 # 3 CF F5 75 Ax MacBook Air 894 $ 5olo % Λ 6 > W F6 K F7 &arrow_forwardConsider this step in a radical reaction: Y What type of step is this? Check all that apply. Draw the products of the step on the right-hand side of the drawing area below. If more than one set of products is possible, draw any set. Also, draw the mechanism arrows on the left-hand side of the drawing area to show how this happens. ionization propagation initialization passivation none of the abovearrow_forward22.16 The following groups are ortho-para directors. (a) -C=CH₂ H (d) -Br (b) -NH2 (c) -OCHS Draw a contributing structure for the resonance-stabilized cation formed during elec- trophilic aromatic substitution that shows the role of each group in stabilizing the intermediate by further delocalizing its positive charge. 22.17 Predict the major product or products from treatment of each compound with Cl₁/FeCl₂- OH (b) NO2 CHO 22.18 How do you account for the fact that phenyl acetate is less reactive toward electro- philic aromatic substitution than anisole? Phenyl acetate Anisole CH (d)arrow_forward
- Show how to convert ethyl benzene to (a) 2,5-dichlorobenzoic acid and (b) 2,4-dichlorobenzoic acid.arrow_forwardHelp me solve this problem. Thank you in advance.arrow_forward22.7 Predict the monoalkylated products of the following reactions with benzene. (a) AlCl3 Ya (b) AlCl3 (c) H3PO4 (d) 22.8 Think-Pair-Share AICI3 The reaction below is a common electrophilic aromatic substitution. SO3 H₂SO4 SO₂H (a) Draw the reaction mechanism for this reaction using HSO,+ as the electrophile. (b) Sketch the reaction coordinate diagram, where the product is lower in energy than the starting reactant. (c) Which step in the reaction mechanism is highest in energy? Explain. (d) Which of the following reaction conditions could be used in an electrophilic aro- matic substitution with benzene to provide substituted phenyl derivatives? (i) AICI3 HNO3 H₂SO4 K2Cr2O7 (iii) H₂SO4 (iv) H₂PO₁arrow_forward
- Is an acid-base reaction the only type of reaction that would cause leavening products to rise?arrow_forwardHelp me understand this! Thank you in advance.arrow_forward22.22 For each compound, indicate which group on the ring is more strongly activating and then draw a structural formula of the major product formed by nitration of the compound. Br CHO (a) CH3 (b) (c) CHO CH3 SO₂H (d) ☑ OCHS NO₂ (e) (f) CO₂H NHCOCH3 NHCOCH, (h) CHS 22.23 The following molecules each contain two aromatic rings. (b) 000-100- H3C (a) (c) Which ring in each undergoes electrophilic aromatic substitution more readily? Draw the major product formed on nitration.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

