
Organic Chemistry
4th Edition
ISBN: 9780073402772
Author: Janice G. Smith
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Question
Chapter 16, Problem 16.41P
Interpretation Introduction
Interpretation: The given diene is to be ranked in increasing order of heat of hydrogenation.
Concept introduction: Diene is a hydrocarbon that contains two
Conjugated diene consists of two double bonds that are separated by a single bond, whereas isolated diene also consists of two double bond but they are separated by two more carbon atoms.
The example that shows the basic difference between conjugated diene and isolated diene is shown below.
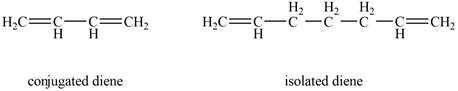
Figure 1
The stability of conjugated diene is more than isolated diene. Due to which the heat of hydrogenation is smaller for conjugated diene, whereas heat of hydrogenation for isolated diene is larger.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
7
Comment on the general features of the predicted (extremely simplified) ¹H-
NMR spectrum of lycopene that is provided below.
00
6
57
PPM
3
2
1
0
Indicate the compound formula: dimethyl iodide (propyl) sulfonium.
Pls help asap
Chapter 16 Solutions
Organic Chemistry
Ch. 16 - Prob. 16.1PCh. 16 - Prob. 16.2PCh. 16 - Draw a second resonance structure for each...Ch. 16 - Prob. 16.4PCh. 16 - Prob. 16.5PCh. 16 - Prob. 16.6PCh. 16 - Prob. 16.7PCh. 16 - Determine the hybridization of the labeled atom in...Ch. 16 - Problem 16.10 Draw the structure consistent with...Ch. 16 - Problem 16.11 Neuroprotectin D1 (NPD1) is...
Ch. 16 - Problem 16.12 Using hybridization, predict how the...Ch. 16 - Problem 16.13 Use resonance theory to explain why...Ch. 16 - Prob. 16.13PCh. 16 - Prob. 16.14PCh. 16 - Prob. 16.15PCh. 16 - Problem 16.17 Draw a stepwise mechanism for the...Ch. 16 - Prob. 16.17PCh. 16 - Problem 16.19 Draw the product formed when each...Ch. 16 - Prob. 16.19PCh. 16 - Prob. 16.20PCh. 16 - Rank the following dienophiles in order of...Ch. 16 - Prob. 16.22PCh. 16 - Prob. 16.23PCh. 16 - Prob. 16.24PCh. 16 - Prob. 16.25PCh. 16 - Problem 16.27 Which compound in each pair absorbs...Ch. 16 - Prob. 16.27PCh. 16 - 16.29 Name each diene and state whether the...Ch. 16 - Prob. 16.29PCh. 16 - Prob. 16.30PCh. 16 - Prob. 16.31PCh. 16 - Prob. 16.32PCh. 16 - Prob. 16.33PCh. 16 - Prob. 16.34PCh. 16 - 16.35 Explain why the cyclopentadienide anion A...Ch. 16 - Explain each statement using resonance theory. a....Ch. 16 - 16.37 Draw the structure of each compound.
a. in...Ch. 16 - Draw and name all dienes of molecular formula...Ch. 16 - Prob. 16.39PCh. 16 - 16.39 Label each pair of compounds as...Ch. 16 - Prob. 16.41PCh. 16 - 16.41 Draw the products formed when each compound...Ch. 16 - Prob. 16.43PCh. 16 - 16.43 Treatment of alkenes A and B with gives the...Ch. 16 - 16.44 Draw a stepwise mechanism for the following...Ch. 16 - Prob. 16.46PCh. 16 - 16.46 Explain, with reference to the mechanism,...Ch. 16 - Prob. 16.48PCh. 16 - Prob. 16.49PCh. 16 - Prob. 16.50PCh. 16 - Prob. 16.51PCh. 16 - Prob. 16.52PCh. 16 - Prob. 16.53PCh. 16 - 16.53 Diels–Alder reaction of a monosubstituted...Ch. 16 - Prob. 16.55PCh. 16 - Prob. 16.56PCh. 16 - 16.55 Devise a stepwise synthesis of each compound...Ch. 16 - Prob. 16.58PCh. 16 - 16.57 A transannular Diels–Alder reaction is an...Ch. 16 - Draw a stepwise mechanism for the following...Ch. 16 - Draw the products of each reaction. Indicate the...Ch. 16 - Prob. 16.62PCh. 16 - Prob. 16.63PCh. 16 - Prob. 16.64PCh. 16 - 16.65 The treatment of isoprene with one...Ch. 16 - 16.66 The treatment of with forms B (molecular...Ch. 16 - Rank the following compounds in the order of...Ch. 16 - Prob. 16.68PCh. 16 - Prob. 16.69PCh. 16 - Prob. 16.70PCh. 16 - Prob. 16.71PCh. 16 - Prob. 16.72PCh. 16 - Prob. 16.73PCh. 16 - Prob. 16.74P
Knowledge Booster
Similar questions
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
