
Organic Chemistry
4th Edition
ISBN: 9780073402772
Author: Janice G. Smith
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 16, Problem 16.44P
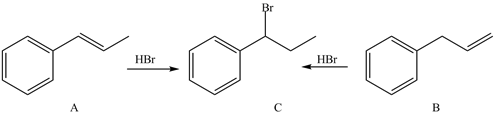
Treatment of
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
When talking about the acidity of carboxylic acids, is it the same thing to say higher or stronger acidity?
Using the following two half-reactions, determine the pH range in which $NO_2^-\ (aq)$ cannot be found as the predominant chemical species in water.* $NO_3^-(aq)+10H^+(aq)+8e^-\rightarrow NH_4^+(aq)+3H_2O(l),\ pE^{\circ}=14.88$* $NO_2^-(aq)+8H^+(aq)+6e^-\rightarrow NH_4^+(aq)+2H_2O(l),\ pE^{\circ}=15.08$
Indicate characteristics of oxodec acid.
Chapter 16 Solutions
Organic Chemistry
Ch. 16 - Prob. 16.1PCh. 16 - Prob. 16.2PCh. 16 - Draw a second resonance structure for each...Ch. 16 - Prob. 16.4PCh. 16 - Prob. 16.5PCh. 16 - Prob. 16.6PCh. 16 - Prob. 16.7PCh. 16 - Determine the hybridization of the labeled atom in...Ch. 16 - Problem 16.10 Draw the structure consistent with...Ch. 16 - Problem 16.11 Neuroprotectin D1 (NPD1) is...
Ch. 16 - Problem 16.12 Using hybridization, predict how the...Ch. 16 - Problem 16.13 Use resonance theory to explain why...Ch. 16 - Prob. 16.13PCh. 16 - Prob. 16.14PCh. 16 - Prob. 16.15PCh. 16 - Problem 16.17 Draw a stepwise mechanism for the...Ch. 16 - Prob. 16.17PCh. 16 - Problem 16.19 Draw the product formed when each...Ch. 16 - Prob. 16.19PCh. 16 - Prob. 16.20PCh. 16 - Rank the following dienophiles in order of...Ch. 16 - Prob. 16.22PCh. 16 - Prob. 16.23PCh. 16 - Prob. 16.24PCh. 16 - Prob. 16.25PCh. 16 - Problem 16.27 Which compound in each pair absorbs...Ch. 16 - Prob. 16.27PCh. 16 - 16.29 Name each diene and state whether the...Ch. 16 - Prob. 16.29PCh. 16 - Prob. 16.30PCh. 16 - Prob. 16.31PCh. 16 - Prob. 16.32PCh. 16 - Prob. 16.33PCh. 16 - Prob. 16.34PCh. 16 - 16.35 Explain why the cyclopentadienide anion A...Ch. 16 - Explain each statement using resonance theory. a....Ch. 16 - 16.37 Draw the structure of each compound.
a. in...Ch. 16 - Draw and name all dienes of molecular formula...Ch. 16 - Prob. 16.39PCh. 16 - 16.39 Label each pair of compounds as...Ch. 16 - Prob. 16.41PCh. 16 - 16.41 Draw the products formed when each compound...Ch. 16 - Prob. 16.43PCh. 16 - 16.43 Treatment of alkenes A and B with gives the...Ch. 16 - 16.44 Draw a stepwise mechanism for the following...Ch. 16 - Prob. 16.46PCh. 16 - 16.46 Explain, with reference to the mechanism,...Ch. 16 - Prob. 16.48PCh. 16 - Prob. 16.49PCh. 16 - Prob. 16.50PCh. 16 - Prob. 16.51PCh. 16 - Prob. 16.52PCh. 16 - Prob. 16.53PCh. 16 - 16.53 Diels–Alder reaction of a monosubstituted...Ch. 16 - Prob. 16.55PCh. 16 - Prob. 16.56PCh. 16 - 16.55 Devise a stepwise synthesis of each compound...Ch. 16 - Prob. 16.58PCh. 16 - 16.57 A transannular Diels–Alder reaction is an...Ch. 16 - Draw a stepwise mechanism for the following...Ch. 16 - Draw the products of each reaction. Indicate the...Ch. 16 - Prob. 16.62PCh. 16 - Prob. 16.63PCh. 16 - Prob. 16.64PCh. 16 - 16.65 The treatment of isoprene with one...Ch. 16 - 16.66 The treatment of with forms B (molecular...Ch. 16 - Rank the following compounds in the order of...Ch. 16 - Prob. 16.68PCh. 16 - Prob. 16.69PCh. 16 - Prob. 16.70PCh. 16 - Prob. 16.71PCh. 16 - Prob. 16.72PCh. 16 - Prob. 16.73PCh. 16 - Prob. 16.74P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the final product when hexanedioic acid reacts with 1º PCl5 and 2º NH3.arrow_forwardWhat is the final product when D-galactose reacts with hydroxylamine?arrow_forwardIndicate the formula of the product obtained by reacting methyl 5-chloro-5-oxopentanoate with 1 mole of 4-penten-1-ylmagnesium bromide.arrow_forward
- The temperature on a sample of pure X held at 1.25 atm and -54. °C is increased until the sample boils. The temperature is then held constant and the pressure is decreased by 0.42 atm. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 2 0 0 200 400 temperature (K) Xarrow_forwardQUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes *The values are all provided in the photo attached*arrow_forwardpressure (atm) 3 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 0 0 200 temperature (K) 400 аarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License