
Concept explainers
Neuroprotectin D1 (NPD1) is synthesized in the body from highly unsaturated essential fatty acids. NPD1 is a potent natural anti-inflammatory agent.
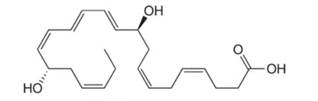
NPD1
a. Label each double bond as conjugated or isolated.
b. Label each double bond as E or Z.
c. For each conjugated system, label the given conformation as s-cis or s-trans.
(a)
Interpretation: Neuroprotectin D1 is synthesized in the body from highly unsaturated essential fatty acids. NPD1 is a potent natural anti-inflammatory agent. The each double bond in NPD1 is to be labeled as conjugated or isolated.
Concept introduction: Diene is a hydrocarbon that contains two
Conjugated diene consists of two double bonds that are separated by a single bond, whereas isolated diene also consists of two double bond but they are separated by two more carbon atoms.
The example that shows the basic difference between conjugated diene and isolated diene is shown below.
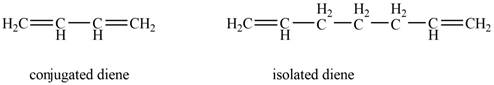
Figure 1
In conjugated diene, electrons in the pi bonds are delocalized, whereas in isolated diene, electrons in the pi bonds are localized.
Answer to Problem 16.10P
The each double bond in NPD1 as conjugated and isolated diene is shown below.
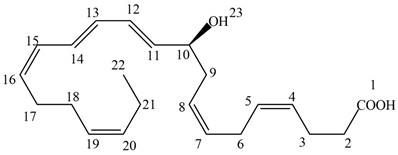
Figure 3
The double bond present between
Explanation of Solution
The given structure of NPD1 is shown below.
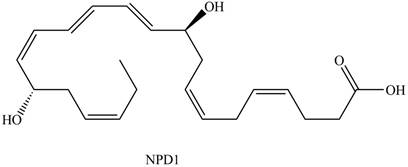
Figure 2
Diene is a hydrocarbon that contains two
Conjugated diene consists of two double bonds that are separated by a single bond, whereas isolated diene also consists of two double bond but they are separated by two more carbon atoms. Therefore, carbon atoms of a conjugated double bond are
The each double bond in NPD1 as conjugated and isolated diene is shown below.
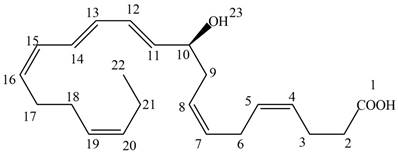
Figure 3
The double bond present between
The each double bond in NPD1 as conjugated or isolated is rightfully stated.
(b)
Interpretation: Neuroprotectin D1 is synthesized in the body from highly unsaturated essential fatty acids. NPD1 is a potent natural anti-inflammatory agent. The each double bond in NPD1 is to be labeled as
Concept introduction: The prefix
Answer to Problem 16.10P
The each double bond in NPD1 as conjugated and isolated diene is shown below.
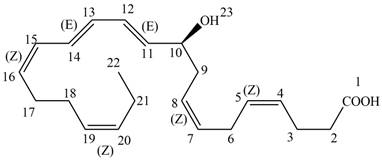
Figure 4
The double bond present between
Explanation of Solution
The given structure of NPD1 is shown below.
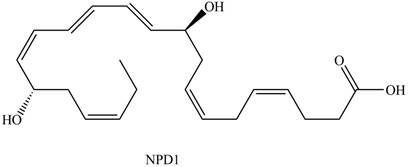
Figure 2
The prefix
The each double bond in NPD1 as conjugated and isolated diene is shown below.
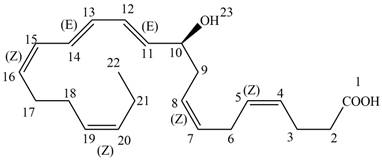
Figure 4
The double bond present between
The each double bond in NPD1 as
(c)
Interpretation: Neuroprotectin D1 is synthesized in the body from highly unsaturated essential fatty acids. NPD1 is a potent natural anti-inflammatory agent. For each conjugated system in NPD1 is to be labeled as
Concept introduction: “If the two double bonds are on the same side of the single bond joining the two double bonds, then it is known as
Answer to Problem 16.10P
The each double bond in NPD1 as conjugated and isolated diene is shown below.
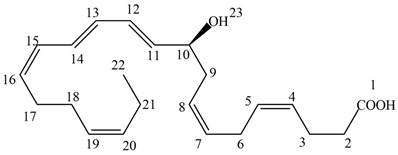
Figure 3
The double bond present between
The double bonds between
Explanation of Solution
The given structure of NPD1 is shown below.
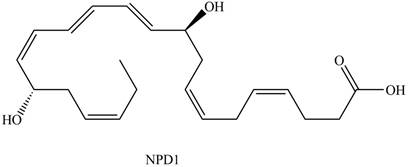
Figure 2
“If the two double bonds are on the same side of the single bond joining the two double bonds, then it is known as
The each double bond in NPD1 as conjugated and isolated diene is shown below.

Figure 3
The double bond present between
The double bonds between
The
Want to see more full solutions like this?
Chapter 16 Solutions
Organic Chemistry
- Using the conditions of spontaneity to deduce the signs of AH and AS Use the observations about each chemical reaction in the table below to decide the sign (positive or negative) of the reaction enthalpy AH and reaction entropy AS. Note: if you have not been given enough information to decide a sign, select the "unknown" option. reaction observations conclusions A The reverse of this reaction is always spontaneous but proceeds faster at temperatures above -48. °C. ΔΗ is (pick one) ✓ AS is (pick one) B This reaction is spontaneous except below 114. °C but proceeds at a slower rate below 135. °C. ΔΗ is (pick one) AS is (pick one) ΔΗ is C This reaction is exothermic and proceeds faster at temperatures above -43. °C. (pick one) AS is (pick one) v Х 5 ? 18 Ararrow_forwardion. A student proposes the following Lewis structure for the perchlorate (CIO) io : :0: : Cl : - - : :0: ك Assign a formal charge to each atom in the student's Lewis structure. atom central O formal charge ☐ top O ☐ right O ☐ bottom O ☐ Cl ☐arrow_forwardDecide whether these proposed Lewis structures are reasonable. proposed Lewis structure Yes. Is the proposed Lewis structure reasonable? Cl- : 2: :Z: :Z: N—N : 0: C C1: O CO No, it has the wrong number of valence electrons. The correct number is: ☐ No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* ☐ Yes. No, it has the wrong number of valence electrons. The correct number is: ☐ No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* | Yes. No, it has the wrong number of valence electrons. The correct number is: No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* | If two or more atoms of the same element don't satisfy the octet rule, just enter the chemical symbol as many times as necessary. For example, if two oxygen atoms don't satisfy the octet rule, enter "0,0". ☑arrow_forward
- Use the observations about each chemical reaction in the table below to decide the sign (positive or negative) of the reaction enthalpy AH and reaction entropy AS. Note: if you have not been given enough information to decide a sign, select the "unknown" option. reaction observations conclusions ΔΗ is (pick one) A This reaction is faster above 103. °C than below. AS is (pick one) ΔΗ is (pick one) B This reaction is spontaneous only above -9. °C. AS is (pick one) ΔΗ is (pick one) C The reverse of this reaction is always spontaneous. AS is (pick one) 18 Ararrow_forwardUse the observations about each chemical reaction in the table below to decide the sign (positive or negative) of the reaction enthalpy AH and reaction entropy AS. Note: if you have not been given enough information to decide a sign, select the "unknown" option. reaction observations conclusions A The reverse of this reaction is always spontaneous but proceeds slower at temperatures below 41. °C. ΔΗ is (pick one) AS is (pick one) ΔΗ is (pick one) B This reaction is spontaneous except above 94. °C. AS is (pick one) This reaction is always spontaneous, but ΔΗ is (pick one) C proceeds slower at temperatures below −14. °C. AS is (pick one) Х 00. 18 Ar 무ㅎ B 1 1arrow_forwardDraw the product of the reaction shown below. Ignore inorganic byproducts. + H CH3CH2OH HCI Drawingarrow_forward
- please explain this in simple termsarrow_forwardK Most Reactive Na (3 pts) Can the metal activity series (shown on the right) or a standard reduction potential table explain why potassium metal can be prepared from the reaction of molten KCI and Na metal but sodium metal is not prepared from the reaction of molten NaCl and K metal? Show how (not). Ca Mg Al с Zn Fe Sn Pb H Cu Ag Au Least Reactivearrow_forward(2 pts) Why is O2 more stable as a diatomic molecule than S2?arrow_forward
- Draw the Lewis structure for the polyatomic phosphite (PO¾³¯) a anion. Be sure to include all resonance structures that satisfy the octet rule. C I A [ ]¯arrow_forwardDecide whether these proposed Lewis structures are reasonable. proposed Lewis structure Is the proposed Lewis structure reasonable? Yes. :0: Cl C C1: 0=0: : 0 : : 0 : H C N No, it has the wrong number of valence electrons. The correct number is: ☐ No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* ☐ Yes. No, it has the wrong number of valence electrons. The correct number is: ☐ No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* Yes. ☐ No, it has the wrong number of valence electrons. The correct number is: ☐ No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* | * If two or more atoms of the same element don't satisfy the octet rule, just enter the chemical symbol as many times as necessary. For example, if two oxygen atoms don't satisfy the octet rule, enter "0,0".arrow_forwardDraw the Lewis structure for the polyatomic trisulfide anion. Be sure to include all resonance structures that satisfy the octet rule. с [ ] - Garrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
