
Interpretation : The relationship between molarity and freezing point depression is to be outlined.
Concept Introduction : Molarity mainly explains the concentration of a solute and the freezing point depression is a colligative property that depends on the number of solute particles and it increases with an increase in the concentration of solute Molarity is the ratio of moles of a solute to the volume of solution in liter. Molarity is the ratio of moles of a solute to the volume of solution in a liter. It is denoted by
The expression for molarity is as follows:
Answer to Problem 12STP
The Freezing point depression is a colligative property that depends on the number of solute particles and it increases with an increase in the concentration of solute as the more the solute more it will alter the freezing point of the solvent or solution.
Explanation of Solution
Molarity mainly explains the concentration of a solute and the freezing point depression is a colligative property that depends on the number of solute particles it increases with an increase in the concentration of solute as the more the solute more it will alter the freezing point of the solvent or solution. Also from the graph, it is clear that freezing point depression increases with an increase in molarity.
The data is given as:
| Molarity | Freezing point depression | ||
The required graph is plotted as:
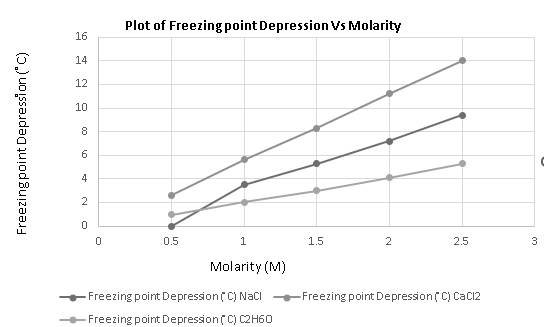
Hence, the freezing point depression increases with an increase in molarity.
The freezing point depression of a solute increases with an increase in molarity.
Chapter 16 Solutions
Chemistry 2012 Student Edition (hard Cover) Grade 11
- Add conditions above and below the arrow that turn the reactant below into the product below in a single transformationADS fint anditions 百 Abl res condinese NC ง Add on condtions 1.0 B H,N.arrow_forward3. Provide all the steps and reagents for this synthesis. OHarrow_forwardSteps and explanationarrow_forward
- Steps and explanations please.arrow_forwardSteps on how to solve. Thank you!arrow_forward3. Name this ether correctly. H₁C H3C CH3 CH3 4. Show the best way to make the ether in #3 by a Williamson Ether Synthesis. Start from an alcohol or phenol. 5. Draw the structure of an example of a sulfide.arrow_forward
- 1. Which one(s) of these can be oxidized with CrO3 ? (could be more than one) a) triphenylmethanol b) 2-pentanol c) Ethyl alcohol d) CH3 2. Write in all the product(s) of this reaction. Label them as "major" or "minor". 2-methyl-2-hexanol H2SO4, heatarrow_forward3) Determine if the pairs are constitutional isomers, enantiomers, diastereomers, or mesocompounds. (4 points)arrow_forwardIn the decomposition reaction in solution B → C, only species C absorbs UV radiation, but neither B nor the solvent absorbs. If we call At the absorbance measured at any time, A0 the absorbance at the beginning of the reaction, and A∞ the absorbance at the end of the reaction, which of the expressions is valid? We assume that Beer's law is fulfilled.arrow_forward
- > You are trying to decide if there is a single reagent you can add that will make the following synthesis possible without any other major side products: 1. ☑ CI 2. H3O+ O Draw the missing reagent X you think will make this synthesis work in the drawing area below. If there is no reagent that will make your desired product in good yield or without complications, just check the box under the drawing area and leave it blank. Click and drag to start drawing a structure. Explanation Check ? DO 18 Ar B © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibilityarrow_forwardDon't use ai to answer I will report you answerarrow_forwardConsider a solution of 0.00304 moles of 4-nitrobenzoic acid (pKa = 3.442) dissolved in 25 mL water and titrated with 0.0991 M NaOH. Calculate the pH at the equivalence pointarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





