
(a)
Interpretation: The species which are used for the transesterification of esters other than acids has to be identified.
Concept introduction:
Transesterification is the process of formation of a new ester molecule from the reaction of alcohol and an ester. This is like hydrolysis of ester but here nucleophile is alcohol molecule instead of
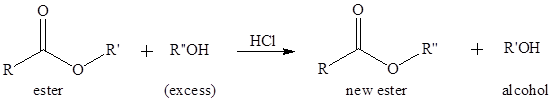
If a better nucleophile is used in place of alcohol molecule that will also increase the rate of transesterification reaction. The conjugate base of an alcohol is an alkoxide which is a better nucleophile and can attack the carbonyl carbon even its oxygen is not protonated.
(b)
Interpretation: The rate of aminolysis reaction of esters does not increase in presence of
Concept introduction: The reaction of a
Want to see the full answer?
Check out a sample textbook solution
Chapter 15 Solutions
ORGANIC CHEMISTRY-W/S.G+SOLN.MANUAL
- can someone draw out the reaction mechanism for this reaction showing all bonds, intermediates and side products Comment on the general features of the 1H-NMR spectrum of isoamyl ester provided belowarrow_forwardWhat would be the best choices for the missing reagents 1 and 3 in this synthesis? 1. PPh3 3 2. n-BuLi • Draw the missing reagents in the drawing area below. You can draw them in any arrangement you like. • Do not draw the missing reagent 2. If you draw 1 correctly, we'll know what it is. • Note: if one of your reagents needs to contain a halogen, use bromine. Click and drag to start drawing a structure.arrow_forwardIdentify the missing organic reactants in the following reaction: X + Y H+ two steps Note: This chemical equation only focuses on the important organic molecules in the reaction. Additional inorganic or small-molecule reactants or products (like H2O) are not shown. In the drawing area below, draw the skeletal ("line") structures of the missing organic reactants X and Y. You may draw the structures in any arrangement that you like, so long as they aren't touching. Click and drag to start drawing a structure. Х :arrow_forward
- I am struggling with the IUPAC (sys H Reply ☑Mark as Unreadarrow_forwardDon't used hand raiting and don't used Ai solution and correct answerarrow_forwardH R Part: 1/2 :CI: is a/an electrophile Part 2 of 2 Draw the skeletal structure of the product(s) for the Lewis acid-base reaction. Include lone pairs and formal charges (if applicable) on the structures. 4-7: H ö- H Skip Part Check X :C1: $ % L Fi Click and drag to start drawing a structure. MacBook Pro & ㅁ x G 0: P Add or increase positive formal cha Save For Later Submit ©2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Centearrow_forward
- Draw the friedel-crafts acylation mechanism of m-Xylenearrow_forwardDon't used hand raiting and don't used Ai solutionarrow_forward1. Base on this experimental results, how do you know that the product which you are turning in is methyl 3-nitrobenzoate(meta substituted product ) rather than either of the other two products? 2. What observation suggests that at least a small amount of one or both of the other two isomers are in the mother liquor?arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

