
Organic Chemistry
12th Edition
ISBN: 9781118875766
Author: T. W. Graham Solomons, Craig B. Fryhle, Scott A. Snyder
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 15, Problem 59P
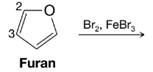
Furan undergoes electrophilic
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Using the following two half-reactions, determine the pH range in which $NO_2^-\ (aq)$ cannot be found as the predominant chemical species in water.* $NO_3^-(aq)+10H^+(aq)+8e^-\rightarrow NH_4^+(aq)+3H_2O(l),\ pE^{\circ}=14.88$* $NO_2^-(aq)+8H^+(aq)+6e^-\rightarrow NH_4^+(aq)+2H_2O(l),\ pE^{\circ}=15.08$
Indicate characteristics of oxodec acid.
What is the final product when hexanedioic acid reacts with 1º PCl5 and 2º NH3.
Chapter 15 Solutions
Organic Chemistry
Ch. 15 - PRACTICE PROBLEM 15.1
Show how loss of a proton...Ch. 15 - Prob. 2PPCh. 15 - PRACTICE PROBLEM 15.3
Outline all steps in a...Ch. 15 - PRACTICE PROBLEM 15.4 Provide a mechanism that...Ch. 15 - Prob. 5PPCh. 15 - Prob. 6PPCh. 15 - Prob. 7PPCh. 15 - PRACTICE PROBLEM 15.8 Write resonance structures...Ch. 15 - PRACTICE PROBLEM 15.9
Provide a mechanism for the...Ch. 15 - PRACTICE PROBLEM 15.10 The trifluoromethyl group...
Ch. 15 - PRACTICE PROBLEM 15.11
Predict the major products...Ch. 15 - PRACTICE PROBLEM 15.12 Predict the major product...Ch. 15 - PRACTICE PROBLEM 15.13
Write mechanisms for the...Ch. 15 - Prob. 14PPCh. 15 - PRACTICE PROBLEM 15.15
Suppose you needed to...Ch. 15 - PRACTICE PROBLEM 15.16 1-Fluoro-2,4-dinitrobenzene...Ch. 15 - Prob. 17PPCh. 15 - PRACTICE PROBLEM 15.18
When...Ch. 15 - PRACTICE PROBLEM 15.19 Birch reduction of toluene...Ch. 15 - Prob. 20PCh. 15 - Prob. 21PCh. 15 - What monobromination product (or products) would...Ch. 15 - 15.23 Predict the major products of the following...Ch. 15 - Prob. 24PCh. 15 - 15.25 Starting with styrene, outline a synthesis...Ch. 15 - Prob. 26PCh. 15 - 15.27 Starting with aniline, outline a synthesis...Ch. 15 - Prob. 28PCh. 15 - Propose structures for compounds GI:Ch. 15 - 2,6-Dichlorophenol has been isolated from the...Ch. 15 - Prob. 31PCh. 15 - 15.32 Give structures (including stereochemistry...Ch. 15 - Provide a detailed mechanism for each of the...Ch. 15 - 15.34 Provide a detailed mechanism for the...Ch. 15 - Prob. 35PCh. 15 - Many polycyclic aromatic compounds have been...Ch. 15 - Prob. 37PCh. 15 - Prob. 38PCh. 15 - Prob. 39PCh. 15 - Prob. 40PCh. 15 - Predict the product of the following reaction.Ch. 15 - 15.42 When m-chlorotoluene is treated with sodium...Ch. 15 - Prob. 43PCh. 15 - Prob. 44PCh. 15 - Prob. 45PCh. 15 - Prob. 46PCh. 15 - 15.47 Provide structures for compounds A and B:
Ch. 15 - Prob. 48PCh. 15 - 15.49 Treating cyclohexene with acetyl chloride...Ch. 15 - 15.50 The tert-butyl group can be used as a...Ch. 15 - 15.51 When toluene is sulfonated (concentrated )...Ch. 15 - Prob. 52PCh. 15 - 2-Methylnaphthalene can be synthesized from...Ch. 15 - Prob. 54PCh. 15 - Prob. 55PCh. 15 - Prob. 56PCh. 15 - Prob. 57PCh. 15 - Prob. 58PCh. 15 - Furan undergoes electrophilic aromatic...Ch. 15 - A C-D bond is harder to break than a C-H bond,...Ch. 15 - 15.61 Acetanilide was subjected to the following...Ch. 15 - Prob. 62PCh. 15 - Prob. 63PCh. 15 - Prob. 64PCh. 15 - When compound C, which is often used to model a...Ch. 15 - Open the molecular model file for benzyne and...Ch. 15 - The structure of thyroxine, a thyroid hormone that...Ch. 15 - Prob. 2LGPCh. 15 - 3. Deduce the structures of compounds E–L in the...
Additional Science Textbook Solutions
Find more solutions based on key concepts
All of the following processes are involved in the carbon cycle except: a. photosynthesis b. cell respiration c...
Human Biology: Concepts and Current Issues (8th Edition)
Name the components (including muscles) of the thoracic cage. List the contents of the thorax.
Human Physiology: An Integrated Approach (8th Edition)
Which culture produces the most lactic acid? Use the following choices to answer questions. a. E. coli growing ...
Microbiology: An Introduction
Draw the structure of the monomer or monomers used to synthesize the following polymers, and indicate whether e...
Organic Chemistry (8th Edition)
61. What is the pH of a solution in which 224 mL of HCl(g), measured at 27.2 °C and 1.02 atm, is dissolved in 1...
Chemistry: A Molecular Approach (4th Edition)
47. Balance each chemical equation.
a.
b.
c.
d.
Introductory Chemistry (6th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the final product when D-galactose reacts with hydroxylamine?arrow_forwardIndicate the formula of the product obtained by reacting methyl 5-chloro-5-oxopentanoate with 1 mole of 4-penten-1-ylmagnesium bromide.arrow_forwardIn the two chair conformations of glucose, the most stable is the one with all the OH groups in the equatorial position. Is this correct?arrow_forward
- please help me with my homeworkarrow_forwardhelparrow_forwardThe temperature on a sample of pure X held at 1.25 atm and -54. °C is increased until the sample boils. The temperature is then held constant and the pressure is decreased by 0.42 atm. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 2 0 0 200 400 temperature (K) Xarrow_forward
- QUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes *The values are all provided in the photo attached*arrow_forwardpressure (atm) 3 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 0 0 200 temperature (K) 400 аarrow_forwarder your payment details | bar xb Home | bartleby x + aleksogi/x/isl.exe/1o u-lgNskr7j8P3jH-1Qs_pBanHhviTCeeBZbufuBYT0Hz7m7D3ZcW81NC1d8Kzb4srFik1OUFhKMUXzhGpw7k1 O States of Matter Sketching a described thermodynamic change on a phase diagram 0/5 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 1 3- 0- 0 200 Explanation Check temperature (K) 400 X Q Search L G 2025 McGraw Hill LLC. All Rights Reserved Terms of Use Privacy Cearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Coenzymes and cofactors; Author: CH15 SWAYAM Prabha IIT Madras;https://www.youtube.com/watch?v=bubY2Nm7hVM;License: Standard YouTube License, CC-BY
Aromaticity and Huckel's Rule; Author: Professor Dave Explains;https://www.youtube.com/watch?v=7-BguH4_WBQ;License: Standard Youtube License