
Organic Chemistry
12th Edition
ISBN: 9781118875766
Author: T. W. Graham Solomons, Craig B. Fryhle, Scott A. Snyder
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 15, Problem 29P
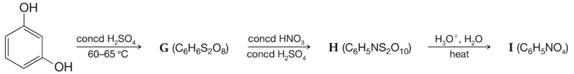
Propose structures for compounds G–I:
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
I need help with the following
I need help with the following
For Raman spectroscopy/imaging, which statement is not true regarding its disadvantages?
a) Limited spatial resolution.
b) Short integration time.
c) A one-dimensional technique.
d) Weak signal, only 1 in 108 incident photons is Raman scattered.
e) Fluorescence interference.
Chapter 15 Solutions
Organic Chemistry
Ch. 15 - PRACTICE PROBLEM 15.1
Show how loss of a proton...Ch. 15 - Prob. 2PPCh. 15 - PRACTICE PROBLEM 15.3
Outline all steps in a...Ch. 15 - PRACTICE PROBLEM 15.4 Provide a mechanism that...Ch. 15 - Prob. 5PPCh. 15 - Prob. 6PPCh. 15 - Prob. 7PPCh. 15 - PRACTICE PROBLEM 15.8 Write resonance structures...Ch. 15 - PRACTICE PROBLEM 15.9
Provide a mechanism for the...Ch. 15 - PRACTICE PROBLEM 15.10 The trifluoromethyl group...
Ch. 15 - PRACTICE PROBLEM 15.11
Predict the major products...Ch. 15 - PRACTICE PROBLEM 15.12 Predict the major product...Ch. 15 - PRACTICE PROBLEM 15.13
Write mechanisms for the...Ch. 15 - Prob. 14PPCh. 15 - PRACTICE PROBLEM 15.15
Suppose you needed to...Ch. 15 - PRACTICE PROBLEM 15.16 1-Fluoro-2,4-dinitrobenzene...Ch. 15 - Prob. 17PPCh. 15 - PRACTICE PROBLEM 15.18
When...Ch. 15 - PRACTICE PROBLEM 15.19 Birch reduction of toluene...Ch. 15 - Prob. 20PCh. 15 - Prob. 21PCh. 15 - What monobromination product (or products) would...Ch. 15 - 15.23 Predict the major products of the following...Ch. 15 - Prob. 24PCh. 15 - 15.25 Starting with styrene, outline a synthesis...Ch. 15 - Prob. 26PCh. 15 - 15.27 Starting with aniline, outline a synthesis...Ch. 15 - Prob. 28PCh. 15 - Propose structures for compounds GI:Ch. 15 - 2,6-Dichlorophenol has been isolated from the...Ch. 15 - Prob. 31PCh. 15 - 15.32 Give structures (including stereochemistry...Ch. 15 - Provide a detailed mechanism for each of the...Ch. 15 - 15.34 Provide a detailed mechanism for the...Ch. 15 - Prob. 35PCh. 15 - Many polycyclic aromatic compounds have been...Ch. 15 - Prob. 37PCh. 15 - Prob. 38PCh. 15 - Prob. 39PCh. 15 - Prob. 40PCh. 15 - Predict the product of the following reaction.Ch. 15 - 15.42 When m-chlorotoluene is treated with sodium...Ch. 15 - Prob. 43PCh. 15 - Prob. 44PCh. 15 - Prob. 45PCh. 15 - Prob. 46PCh. 15 - 15.47 Provide structures for compounds A and B:
Ch. 15 - Prob. 48PCh. 15 - 15.49 Treating cyclohexene with acetyl chloride...Ch. 15 - 15.50 The tert-butyl group can be used as a...Ch. 15 - 15.51 When toluene is sulfonated (concentrated )...Ch. 15 - Prob. 52PCh. 15 - 2-Methylnaphthalene can be synthesized from...Ch. 15 - Prob. 54PCh. 15 - Prob. 55PCh. 15 - Prob. 56PCh. 15 - Prob. 57PCh. 15 - Prob. 58PCh. 15 - Furan undergoes electrophilic aromatic...Ch. 15 - A C-D bond is harder to break than a C-H bond,...Ch. 15 - 15.61 Acetanilide was subjected to the following...Ch. 15 - Prob. 62PCh. 15 - Prob. 63PCh. 15 - Prob. 64PCh. 15 - When compound C, which is often used to model a...Ch. 15 - Open the molecular model file for benzyne and...Ch. 15 - The structure of thyroxine, a thyroid hormone that...Ch. 15 - Prob. 2LGPCh. 15 - 3. Deduce the structures of compounds E–L in the...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Give a molecular orbital description for each of the following: a. 1,3-pentadiene b. 1,4-pentadiene c. 1,3,5-he...
Organic Chemistry (8th Edition)
Body, Heal Thyself The precision of mitotic cell division is essential for repairing damaged tissues like those...
Biology: Life on Earth with Physiology (11th Edition)
3. Which of the following is a major functional characteristic of all organisms? (a) movement, (b) growth (c) m...
Human Anatomy & Physiology (Marieb, Human Anatomy & Physiology) Standalone Book
61. What is the pH of a solution in which 224 mL of HCl(g), measured at 27.2 °C and 1.02 atm, is dissolved in 1...
Chemistry: A Molecular Approach (4th Edition)
Which culture produces the most lactic acid? Use the following choices to answer questions. a. E. coli growing ...
Microbiology: An Introduction
Match each of the following items with all the terms it applies to:
Human Physiology: An Integrated Approach (8th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Using a cell of known pathlength b = 1.25115 x 10-3 cm, a water absorption spectrum was measured. The band at 1645 cm-1, assigned to the O-H bending, showed an absorbance, A, of 1.40. a) Assuming that water density is 1.00 g/mL, calculate the water molar concentration c (hint: M= mole/L) b) Calculate the molar absorptivity, a, of the 1645 cm-1 band c) The transmitted light, I, can be written as I= Ioexp(-xb), where x is the absorption coefficient (sometimes designated as alpha), Io is the input light, and b is the cell pathlength. Prove that x= (ln10)*x*c. (Please provide a full derivation of the equation for x from the equation for I). d) Calculate x for the 1645 cm-1 bandarrow_forwardI need help with the follloaingarrow_forwardFor a CARS experiment on a Raman band 918 cm-1, if omega1= 1280 nm, calculate the omega2 in wavelength (nm) and the CARS output in wavelength (nm).arrow_forward
- I need help with the following questionarrow_forwardFor CARS, which statement is not true regarding its advantages? a) Contrast signal based on vibrational characteristics, no need for fluorescent tagging. b) Stronger signals than spontaneous Raman. c) Suffers from fluorescence interference, because CARS signal is at high frequency. d) Faster, more efficient imaging for real-time analysis. e) Higher resolution than spontaneous Raman microscopy.arrow_forwardDraw the major product of the Claisen condensation reaction between two molecules of this ester. Ignore inorganic byproducts. Incorrect, 5 attempts remaining 1. NaOCH3/CH3OH 2. Acidic workup Select to Draw O Incorrect, 5 attempts remaining The total number of carbons in the parent chain is incorrect. Review the reaction conditions including starting materials and/or intermediate structures and recount the number of carbon atoms in the parent chain of your structure. OKarrow_forward
- Using a cell of known pathlength b = 1.25115 x 10-3 cm, a water absorption spectrum was measured. The band at 1645 cm-1, assigned to the O-H bending, showed an absorbance, A, of 1.40. a) Assuming that water density is 1.00 g/mL, calculate the water molar concentration c (hint: M= mole/L) b) Calculate the molar absorptivity, a, of the 1645 cm-1 band c) The transmitted light, I, can be written as I= Ioexp(-xb), where x is the absorption coefficient (sometimes designated as alpha), Io is the input light, and b is the cell pathlength. Prove that x= (ln10)*x*c d) Calculate x for the 1645 cm-1 bandarrow_forwardConvert 1.38 eV into wavelength (nm) and wavenumber (cm-1) (c = 2.998 x 108 m/s; h = 6.626 x 10-34 J*s).arrow_forwardCan you help me understand the CBC method on metal bridging by looking at this problem?arrow_forward
- A partir de Aluminio y Co(NO3)2ꞏ6H2O, indicar las reacciones a realizar para obtener Azul de Thenard (Al2CoO4).arrow_forwardTo obtain Thenard Blue (Al2CoO4), the following reaction is correct (performed in an oven):Al(OH)3 + Co(OH)2 → Al2CoO4 + 4 H2Oarrow_forwardProblem 38 can u explain and solve thanks april 24arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Coenzymes and cofactors; Author: CH15 SWAYAM Prabha IIT Madras;https://www.youtube.com/watch?v=bubY2Nm7hVM;License: Standard YouTube License, CC-BY
Aromaticity and Huckel's Rule; Author: Professor Dave Explains;https://www.youtube.com/watch?v=7-BguH4_WBQ;License: Standard Youtube License