
Introduction to General, Organic and Biochemistry
12th Edition
ISBN: 9780357119303
Author: Bettelheim, Frederick A., Brown, William H., Campbell, Mary K., FARRELL, Shawn O., Torres, Omar
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 15, Problem 49P
16-54 Several poisonous plants, including Atropa
belladonna, contain the alkaloid atropine. The name “belladonna” (which means “beautiful lady”) probably comes from the fact that Roman women used extracts from this plant to make themselves more attractive. Atropine is widely used by ophthal mologists and optometrists to dilate the pupils for eye examination.
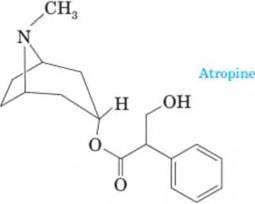
- Classify the amino group in atropine as primary, secondary, or tertiary.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
1.
6. Draw the products for the following reaction:
2.
Diels-Aider
reaction
NOH O
OH
3.
4.
Please correct answer and don't used hand raiting
Chapter 15 Solutions
Introduction to General, Organic and Biochemistry
Ch. 15.1 - Prob. 15.1QCCh. 15.2 - Problem 16-2 Write a structural formula for each...Ch. 15.2 - Prob. 15.3QCCh. 15.3 - Problem 16-4 Select the stronger base from each...Ch. 15.3 - Prob. 15.5QCCh. 15 - 16-6 Answer true or false. te/7-Butylamine is a 3°...Ch. 15 - Prob. 2PCh. 15 - Prob. 3PCh. 15 - 16-9 In what way are pyridine and pyrimidine...Ch. 15 - Prob. 5P
Ch. 15 - Prob. 6PCh. 15 - Prob. 7PCh. 15 - 16-13 Classify each amino group as primary,...Ch. 15 - Prob. 9PCh. 15 - 16-15 There are eight primary amines with the...Ch. 15 - Prob. 11PCh. 15 - 16-17 Propylamine (bp 48°C), ethylmethylamine (bp...Ch. 15 - 16-18 Account for the fact that 1-butanamine (bp...Ch. 15 - 16-19 2-Me thy 1 propane (bp -12°C), 2-propanol...Ch. 15 - Prob. 15PCh. 15 - Prob. 16PCh. 15 - Prob. 17PCh. 15 - Prob. 18PCh. 15 - Prob. 19PCh. 15 - Prob. 20PCh. 15 - 16-26 The p/fb of amphetamine is approximately 3.2...Ch. 15 - 16-27 Guanidine, p/Ca 13.6, is a very strong base,...Ch. 15 - 16-28 Following is the structural formula of...Ch. 15 - Prob. 24PCh. 15 - Prob. 25PCh. 15 - Prob. 26PCh. 15 - 16*32 Many tumors of the breast are correlated...Ch. 15 - Prob. 28PCh. 15 - Prob. 29PCh. 15 - Prob. 30PCh. 15 - (Chemical Connections 15B ) Identify all...Ch. 15 - Prob. 32PCh. 15 - Prob. 33PCh. 15 - Prob. 34PCh. 15 - Prob. 35PCh. 15 - Prob. 36PCh. 15 - (Chemical Connections 15D ) Suppose you saw this...Ch. 15 - Prob. 38PCh. 15 - Prob. 39PCh. 15 - Prob. 40PCh. 15 - 16-46 Arrange these three compounds in order of...Ch. 15 - Prob. 42PCh. 15 - Prob. 43PCh. 15 - Prob. 44PCh. 15 - Prob. 45PCh. 15 - Prob. 46PCh. 15 - Prob. 47PCh. 15 - Prob. 48PCh. 15 - 16-54 Several poisonous plants, including Atropa...Ch. 15 - Prob. 50PCh. 15 - Prob. 51PCh. 15 - Prob. 52PCh. 15 - 16-58 Following is a structural formula of...Ch. 15 - Prob. 54P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Pleasssssseeee solve this question in cheeemsirty, thankss sirarrow_forwardThe Ksp for lead iodide ( Pbl₂) is 1.4 × 10-8. Calculate the solubility of lead iodide in each of the following. a. water Solubility = mol/L b. 0.17 M Pb(NO3)2 Solubility = c. 0.017 M NaI mol/L Solubility = mol/Larrow_forwardPleasssssseeee solve this question in cheeemsirty, thankss sirarrow_forward
- Pleasssssseeee solve this question in cheeemsirty, thankss sirarrow_forwardOnly 100% sure experts solve it correct complete solutions need to get full marks it's my quiz okkkk.take your time but solve full accurate okkk chemistry expert solve itarrow_forwardPleasssssseeee solve this question in cheeemsirty, thankss sirarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning
Acid-Base Titration | Acids, Bases & Alkalis | Chemistry | FuseSchool; Author: FuseSchool - Global Education;https://www.youtube.com/watch?v=yFqx6_Y6c2M;License: Standard YouTube License, CC-BY