
Introduction to General, Organic and Biochemistry
12th Edition
ISBN: 9780357391594
Author: Frederick A. Bettelheim; William H. Brown; Mary K. Campbell
Publisher: Cengage Learning US
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 15, Problem 32P
Interpretation Introduction
Interpretation: The two nitrogen atoms in nicotine should be identified that is converted to its salt by reacting with one mole of
Concept Introduction:The structure of nicotine is:
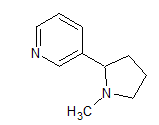
There are two rings, pyridine and pyrrolidine, present in the molecule of nicotine.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Add conditions above and below the arrow that turn the reactant below into the product below in a single transformation.
+ More...
If you need to write reagents above and below the arrow that have complex hydrocarbon groups in them, there is a set of standard abbreviations you can use.
More...
T
H,N
NC
Dat
Indicate the order of basicity of primary, secondary and tertiary amines.
>
Classify each of the following molecules as aromatic, antiaromatic, or nonaromatic.
Cl
Z-
N
O aromatic
O antiaromatic
O nonaromatic
O aromatic
O antiaromatic
O nonaromatic
O aromatic
○ antiaromatic
nonaromatic
Chapter 15 Solutions
Introduction to General, Organic and Biochemistry
Ch. 15.1 - Prob. 15.1QCCh. 15.2 - Problem 16-2 Write a structural formula for each...Ch. 15.2 - Prob. 15.3QCCh. 15.3 - Problem 16-4 Select the stronger base from each...Ch. 15.3 - Prob. 15.5QCCh. 15 - 16-6 Answer true or false. te/7-Butylamine is a 3°...Ch. 15 - Prob. 2PCh. 15 - Prob. 3PCh. 15 - 16-9 In what way are pyridine and pyrimidine...Ch. 15 - Prob. 5P
Ch. 15 - Prob. 6PCh. 15 - Prob. 7PCh. 15 - 16-13 Classify each amino group as primary,...Ch. 15 - Prob. 9PCh. 15 - 16-15 There are eight primary amines with the...Ch. 15 - Prob. 11PCh. 15 - 16-17 Propylamine (bp 48°C), ethylmethylamine (bp...Ch. 15 - 16-18 Account for the fact that 1-butanamine (bp...Ch. 15 - 16-19 2-Me thy 1 propane (bp -12°C), 2-propanol...Ch. 15 - Prob. 15PCh. 15 - Prob. 16PCh. 15 - Prob. 17PCh. 15 - Prob. 18PCh. 15 - Prob. 19PCh. 15 - Prob. 20PCh. 15 - 16-26 The p/fb of amphetamine is approximately 3.2...Ch. 15 - 16-27 Guanidine, p/Ca 13.6, is a very strong base,...Ch. 15 - 16-28 Following is the structural formula of...Ch. 15 - Prob. 24PCh. 15 - Prob. 25PCh. 15 - Prob. 26PCh. 15 - 16*32 Many tumors of the breast are correlated...Ch. 15 - Prob. 28PCh. 15 - Prob. 29PCh. 15 - Prob. 30PCh. 15 - (Chemical Connections 15B ) Identify all...Ch. 15 - Prob. 32PCh. 15 - Prob. 33PCh. 15 - Prob. 34PCh. 15 - Prob. 35PCh. 15 - Prob. 36PCh. 15 - (Chemical Connections 15D ) Suppose you saw this...Ch. 15 - Prob. 38PCh. 15 - Prob. 39PCh. 15 - Prob. 40PCh. 15 - 16-46 Arrange these three compounds in order of...Ch. 15 - Prob. 42PCh. 15 - Prob. 43PCh. 15 - Prob. 44PCh. 15 - Prob. 45PCh. 15 - Prob. 46PCh. 15 - Prob. 47PCh. 15 - Prob. 48PCh. 15 - 16-54 Several poisonous plants, including Atropa...Ch. 15 - Prob. 50PCh. 15 - Prob. 51PCh. 15 - Prob. 52PCh. 15 - 16-58 Following is a structural formula of...Ch. 15 - Prob. 54P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please help me answer this question. I don't understand how or even if this can happen in a single transformation. Please provide a detailed explanation and a drawing showing how it can happen in a single transformation. Add the necessary reagents and reaction conditions above and below the arrow in this organic reaction. If the products can't be made from the reactant with a single transformation, check the box under the drawing area instead.arrow_forward2) Draw the correct chemical structure (using line-angle drawings / "line structures") from their given IUPAC name: a. (E)-1-chloro-3,4,5-trimethylhex-2-ene b. (Z)-4,5,7-trimethyloct-4-en-2-ol C. (2E,6Z)-4-methylocta-2,6-dienearrow_forwardපිපිම Draw curved arrows to represent the flow of electrons in the reaction on the left Label the reactants on the left as either "Acid" or "Base" (iii) Decide which direction the equilibrium arrows will point in each reaction, based on the given pk, values (a) + H-O H 3-H + (c) H" H + H****H 000 44-00 NH₂ (e) i Дон OH Ө NHarrow_forward
- 3) Label the configuration in each of the following alkenes as E, Z, or N/A (for non-stereogenic centers). 00 E 000 N/A E Br N/A N/A (g) E N/A OH E (b) Oz N/A Br (d) 00 E Z N/A E (f) Oz N/A E (h) Z N/Aarrow_forward6) Fill in the missing Acid, pKa value, or conjugate base in the table below: Acid HCI Approximate pK, -7 Conjugate Base H-C: Hydronium (H₂O') -1.75 H-O-H Carboxylic Acids (RCOOH) Ammonium (NH4) 9.24 Water (H₂O) H-O-H Alcohols (ROH) RO-H Alkynes R--H Amines 25 25 38 HOarrow_forward5) Rank the following sets of compounds in order of decreasing acidity (most acidic to least acidic), and choose the justification(s) for each ranking. (a) OH V SH я вон CH most acidic (lowst pKa) least acidic (highest pKa) Effect(s) Effect(s) Effect(s) inductive effect O inductive effect O inductive effect electronegativity electronegativity O electronegativity resonance polarizability resonance polarizability O resonance O polarizability hybridization Ohybridization O hybridization оarrow_forward
- How negatively charged organic bases are formed.arrow_forwardNonearrow_forward1) For the following molecules: (i) Label the indicated alkenes as either cis (Z), trans (E), or N/A (for non-stereogenic centers) by bubbling in the appropriate label on the molecule. (ii) Complete the IUPAC name located below the structure (HINT: Put the letter of the configuration in parentheses at the beginning of the name!) E z N/A ()-3,4,6-trimethylhept-2-ene E Oz O N/A ()-3-ethyl-1-fluoro-4-methylhex-3-ene E -+- N/A Me )-2,3-dimethylpent-2-ene (d) (b) E O N/A Br ()-5-bromo-1-chloro-3-ethyloct-4-ene ОЕ Z N/A Et (___)-3-ethyl-4-methylhex-3-ene E (f) Oz N/A z N/A HO (4.7)-4-(2-hydroxyethyl)-7-methylnona-4,7-dien-2-onearrow_forward
- O 9:21AM Tue Mar 4 ## 64% Problem 51 of 15 Submit Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. H :0: CI. AI :CI: :CI: Cl AI Select to Add Arrows Select to Add Arrows O: Cl :CI: :0: H CI: CI CO Select to Add Arrows Select to Add Arrows :O: CI :0: Cl. 10: AIarrow_forward(i) Draw in the missing lone pair(s) of electrons of the reactants on the left (ii) Draw (curved) arrows to show the flow of electrons in the acid/base reaction on the left (iii) Draw the products of the acid/base on the right (iv) Select the correct label for each product as either "conjugate acid" or "conjugate base" (a) JOH OH NH₂ acid base (b) De "H conjugate acid conjugate acid conjugate base conjugate base acid base conjugate acid conjugate base conjugate acid conjugate base acid basearrow_forwardCould someone answer this NMR and explain please Comment on the general features of the 1H-NMR spectrum of isoamyl ester provided below.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning