
(a)
Interpretation:
The structural formula of missing compound in the given reaction has to be drawn.
Concept Introduction:
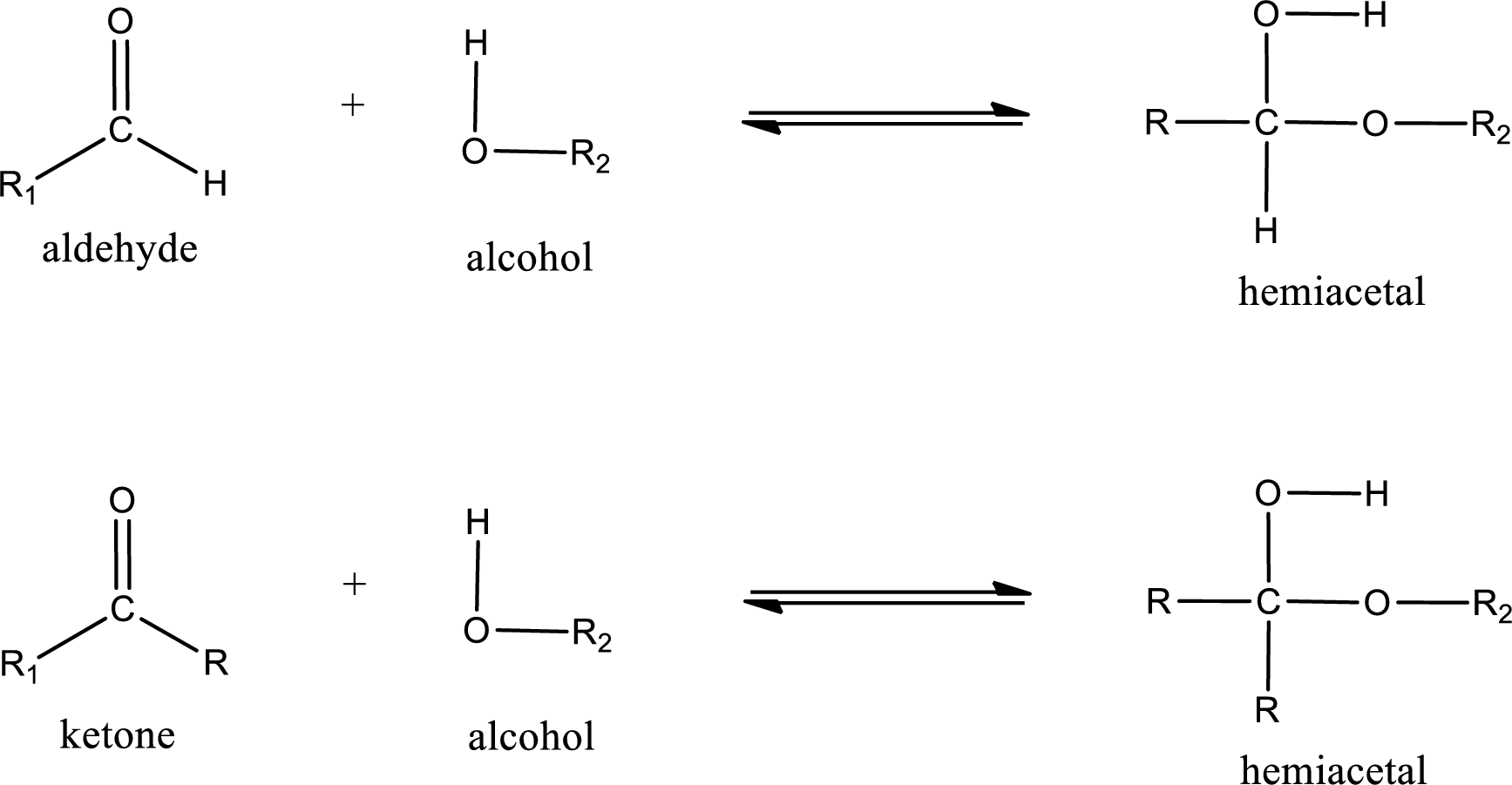
Aldehydes and ketones react with alcohol to form hemiacetal as the product. This reacts with further molecule of aldehyde or ketone to form acetal.
Hemiacetal is an addition product that is obtained by reaction between aldehyde or ketone with alcohol. The general reaction of hemiacetal formation can be given as,
(b)
Interpretation:
The structural formula of missing compound in the given reaction has to be drawn.
Concept Introduction:
Aldehydes contain a carbonyl group that is bonded to a hydrogen atom and a carbon atom. Ketones are compounds that contain a carbonyl group bonded to two carbon atoms. Aldehydes and ketones undergo addition reaction across the carbonyl group.
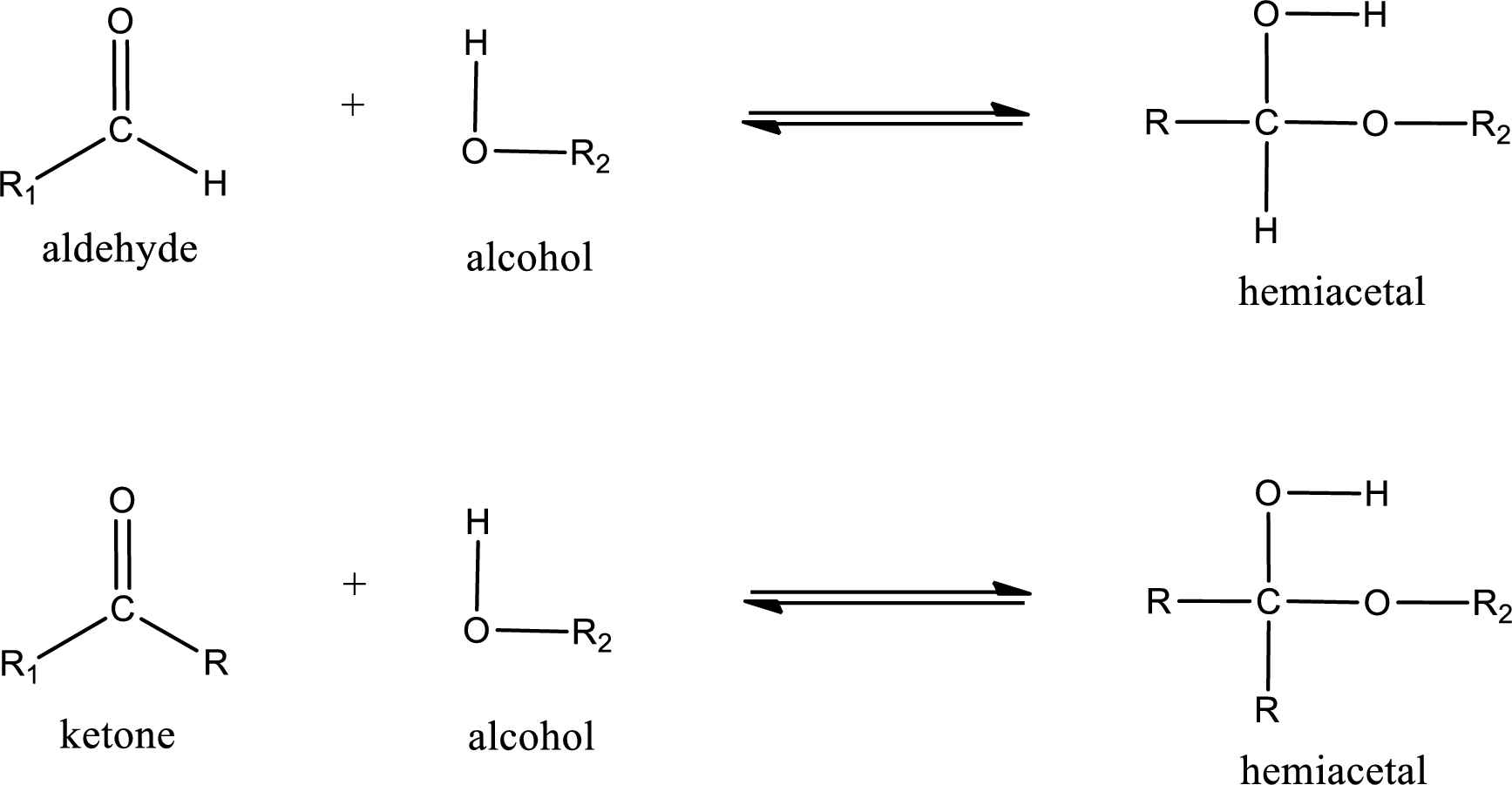
Aldehydes and ketones react with alcohol to form hemiacetal as the product. This reacts with further molecule of aldehyde or ketone to form acetal.
Hemiacetal is an addition product that is obtained by reaction between aldehyde or ketone with alcohol. The general reaction of hemiacetal formation can be given as,
(c)
Interpretation:
The structural formula of missing compound in the given reaction has to be drawn.
Concept Introduction:
Aldehydes contain a carbonyl group that is bonded to a hydrogen atom and a carbon atom. Ketones are compounds that contain a carbonyl group bonded to two carbon atoms. Aldehydes and ketones undergo addition reaction across the carbonyl group.
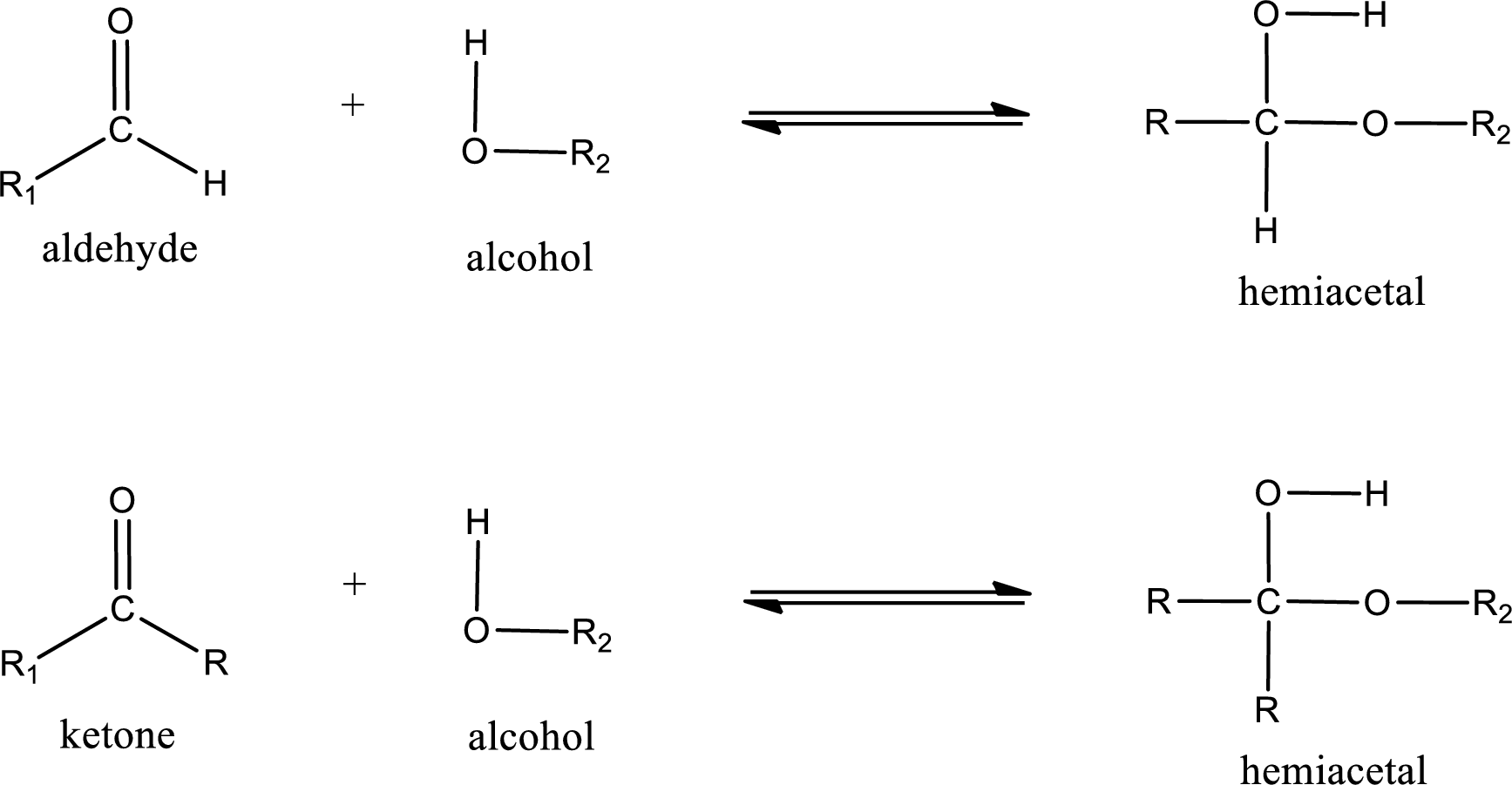
Aldehydes and ketones react with alcohol to form hemiacetal as the product. This reacts with further molecule of aldehyde or ketone to form acetal.
Hemiacetal is an addition product that is obtained by reaction between aldehyde or ketone with alcohol. The general reaction of hemiacetal formation can be given as,
(d)
Interpretation:
The structural formula of missing compound in the given reaction has to be drawn.
Concept Introduction:
Aldehydes contain a carbonyl group that is bonded to a hydrogen atom and a carbon atom. Ketones are compounds that contain a carbonyl group bonded to two carbon atoms. Aldehydes and ketones undergo addition reaction across the carbonyl group.
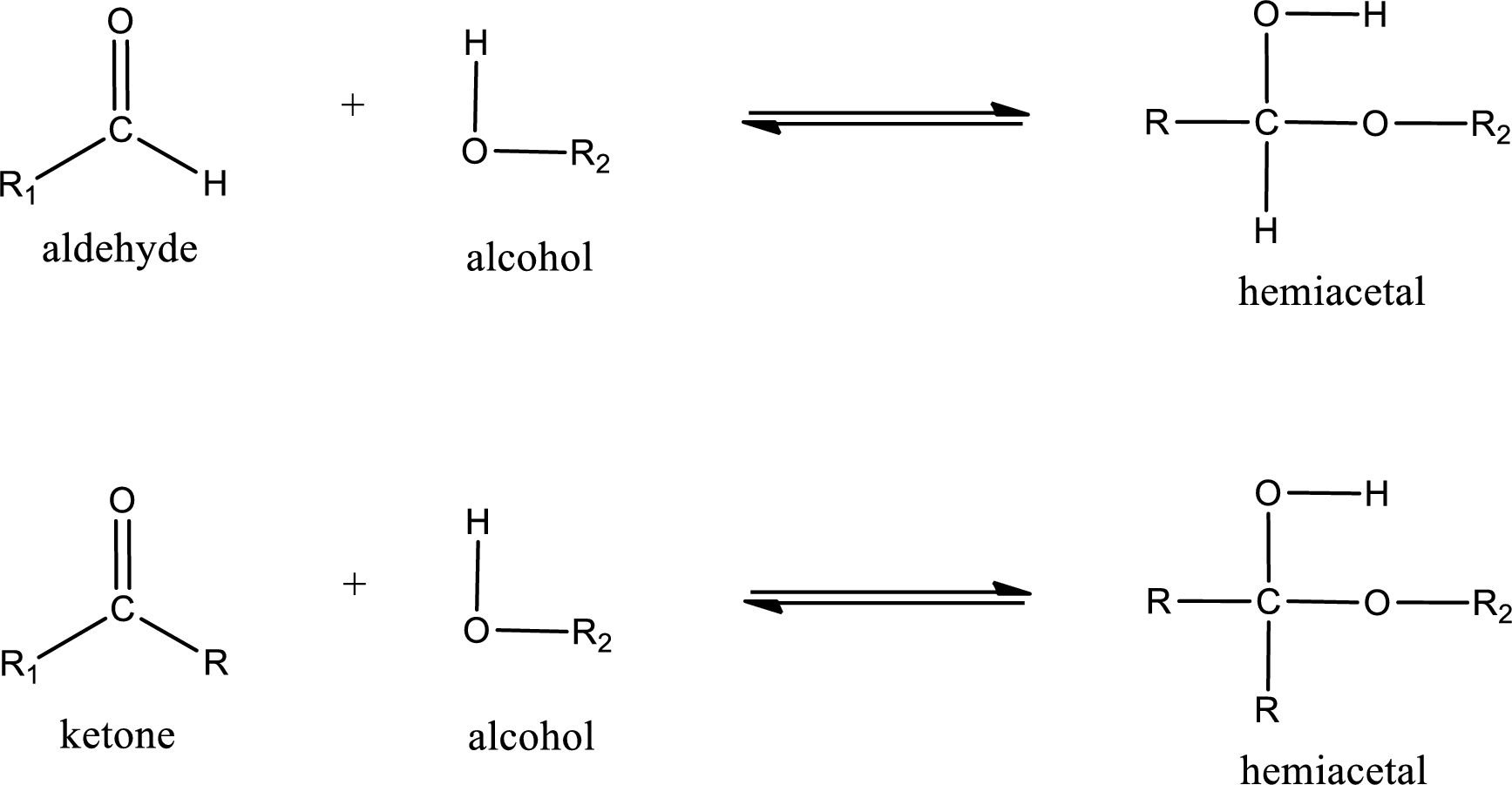
Aldehydes and ketones react with alcohol to form hemiacetal as the product. This reacts with further molecule of aldehyde or ketone to form acetal.
Hemiacetal is an addition product that is obtained by reaction between aldehyde or ketone with alcohol. The general reaction of hemiacetal formation can be given as,
Trending nowThis is a popular solution!

Chapter 15 Solutions
General, Organic, and Biological Chemistry
- in the scope of the SCH4U course! please show all steps as im still learning how to format my answers in the format given, thank you!arrow_forwardhelp me solve this HWarrow_forwardMolecules of the form AH2 can exist in two potential geometries: linear or bent. Construct molecular orbital diagrams for linear and bent CH2. Identify the relevant point group, include all of the appropriate symmetry labels and pictures, and fill in the electrons. Which geometry would you predict to be more stable, and why? (Please draw out the diagram and explain)arrow_forward
- Indicate the variation in conductivity with concentration in solutions of strong electrolytes and weak electrolytes.arrow_forwardThe molar conductivity of a very dilute solution of NaCl has been determined. If it is diluted to one-fourth of the initial concentration, qualitatively explain how the molar conductivity of the new solution will compare with the first.arrow_forwardWhat does the phrase mean, if instead of 1 Faraday of electricity, Q coulombs (Q/F Faradays) pass through?arrow_forward
- What characteristics should an interface that forms an electrode have?arrow_forwardFor a weak acid AcH, calculate the dissociated fraction (alpha), if its concentration is 1.540 mol L-1 and the concentration [H+] is 5.01x10-4 mol L-1.arrow_forwardIf the molar conductivity at infinite dilution of HAC is A0 = 390.5 S cm² mol¹. Calculate the Arrhenius conductivity of a 9.3% by weight solution of HAc with a pH of 3.3. Data: molecular weight of HAC is 60.05 g/mol and the density of the solution is 1 g/cm³.arrow_forward
- If the molar conductivity at infinite dilution of HAC is A0 = 390.5 S cm² mol¹. Calculate the Arrhenius conductivity of a 9.3% by weight solution of HAc with a pH of 3.3. Data: molecular weight of HAC is 60.05 g/mol and the density of the solution is 1 g/cm³.arrow_forwardIf the molar conductivity at infinite dilution of HAC is A0 = 390.5 S cm² mol¹. Calculate the Arrhenius conductivity of a 9.3% by weight solution of HAc with a pH of 3.3. Data: molecular weight of HAC is 60.05 g/mol and the density of the solution is 1 g/cm³.arrow_forwardDetermine the distance between the metal and the OHP layer using the Helm- holtz model when the electrode's differential capacitance is 145 μF cm². DATA: dielectric constant of the medium for the interfacial zone &r= lectric constant of the vacuum &0 = 8.85-10-12 F m-1 = 50, die-arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning





