
Concept explainers
What is the chemical formula of the inorganic product formed, if any, in each of the reactions in Problem 15-78?
- a. Propanal in the Tollens test
- b. 3-Pentanone in the Tollens test
- c. Methylpropanal in the Benedict’s test
- d. Propanone in the Benedict’s test
(a)
Interpretation:
The inorganic product formed when propanal undergoes Tollen’s test has to be given.
Concept Introduction:
In organic chemistry, oxidation reaction is referred to the number
In organic chemistry, reduction reaction is referred to the number
Alcohols undergo oxidation reaction and reduction reaction. This depends upon the number of hydrogen atoms that is bonded to the alpha carbon atom. Primary and secondary alcohol undergoes oxidation reaction while tertiary alcohol does not undergo oxidation reaction. Primary alcohols undergo oxidation to give aldehyde and carboxylic acid as product. Secondary alcohol undergoes oxidation to give ketone as the product.
Aldehyde undergoes oxidation to give carboxylic acid as the product while ketone does not undergo oxidation reaction.
Tollen’s test:
This is also known as silver mirror test. The reagent that is used in Tollen’s test is silver nitrate and ammonia in water. Aldehyde reacts with Tollen’s reagent, where the silver ion is reduced to silver metal and the aldehyde is oxidized to carboxylic acid.
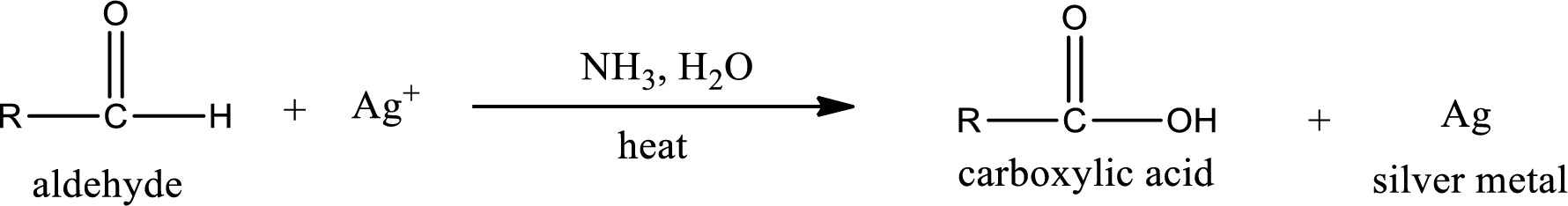
Ketone does not undergo Tollen’s test to deposit silver metal.
Benedict’s test:
This test is also similar to Tollen’s test. In this test,
Answer to Problem 15.80EP
The inorganic product formed is silver metal.
Explanation of Solution
Aldehydes undergo Tollen’s test. The product formed when aldehyde undergo oxidation is a carboxylic acid. The general oxidation reaction for aldehyde can be given as,
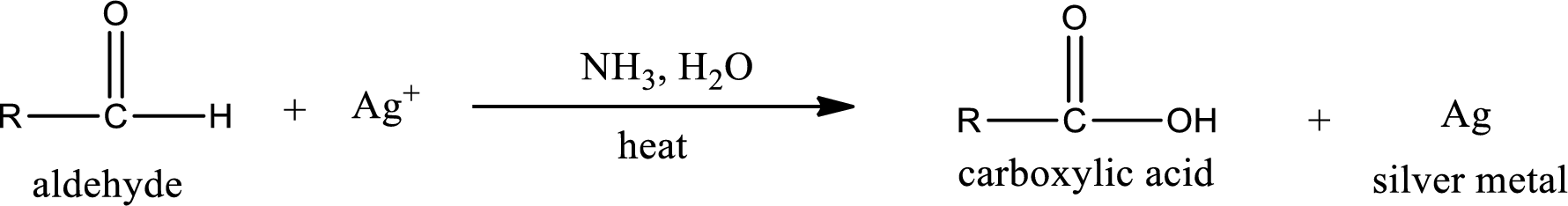
Given aldehyde is propanal and the structure can be given as shown below,
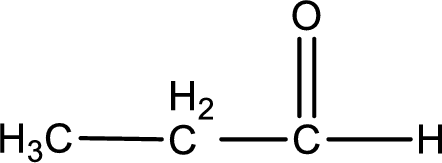
This on reaction with Tollen’s reagent gives carboxylic acid and silver metal as the product. The structure of the inorganic product formed and the complete reaction can be given as shown below,
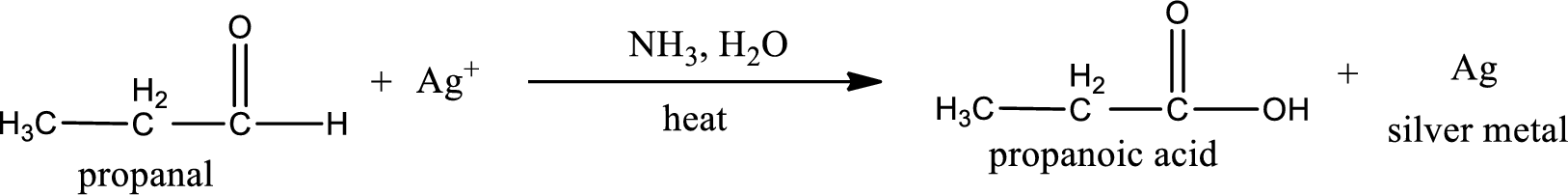
Silver metal is formed as the inorganic product when propanal undergoes Tollen’s test.
The inorganic product formed is given.
(b)
Interpretation:
The inorganic product formed when 3-pentanone undergoes Tollen’s test has to be given.
Concept Introduction:
In organic chemistry, oxidation reaction is referred to the number
In organic chemistry, reduction reaction is referred to the number
Alcohols undergo oxidation reaction and reduction reaction. This depends upon the number of hydrogen atoms that is bonded to the alpha carbon atom. Primary and secondary alcohol undergoes oxidation reaction while tertiary alcohol does not undergo oxidation reaction. Primary alcohols undergo oxidation to give aldehyde and carboxylic acid as product. Secondary alcohol undergoes oxidation to give ketone as the product.
Aldehyde undergoes oxidation to give carboxylic acid as the product while ketone does not undergo oxidation reaction.
Tollen’s test:
This is also known as silver mirror test. The reagent that is used in Tollen’s test is silver nitrate and ammonia in water. Aldehyde reacts with Tollen’s reagent, where the silver ion is reduced to silver metal and the aldehyde is oxidized to carboxylic acid.
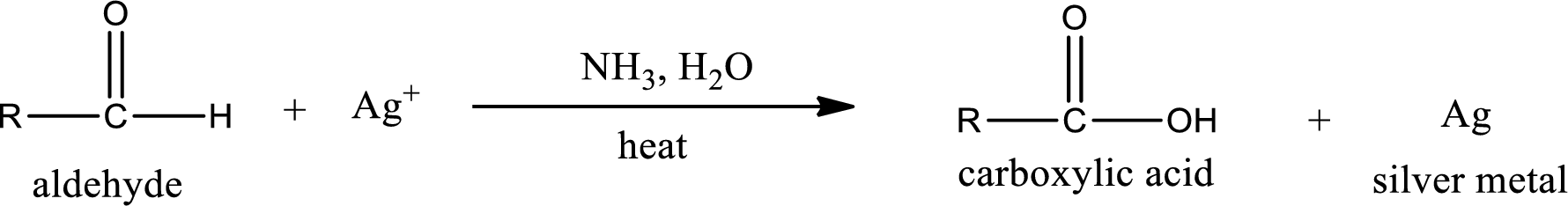
Ketone does not undergo Tollen’s test to deposit silver metal.
Benedict’s test:
This test is also similar to Tollen’s test. In this test,
Answer to Problem 15.80EP
No inorganic product is obtained as 3-pentanone does not undergo Tollen’s test.
Explanation of Solution
Aldehydes undergo Tollen’s test. The product formed when aldehyde undergo oxidation is a carboxylic acid. The general oxidation reaction for aldehyde can be given as,
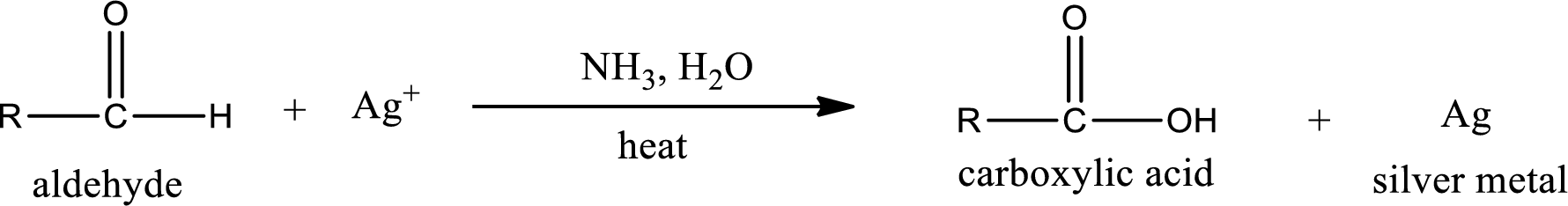
Given compound is a ketone that is 3-pentanone and the structure can be given as shown below,
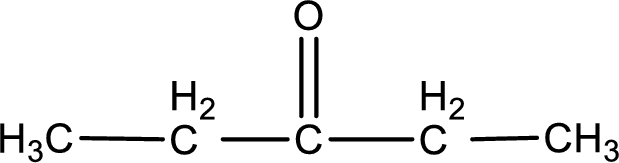
This on reaction with Tollen’s reagent does not give oxidized product. Therefore, no reaction takes place when 3-pentanone reacts with Tollen’s reagent.
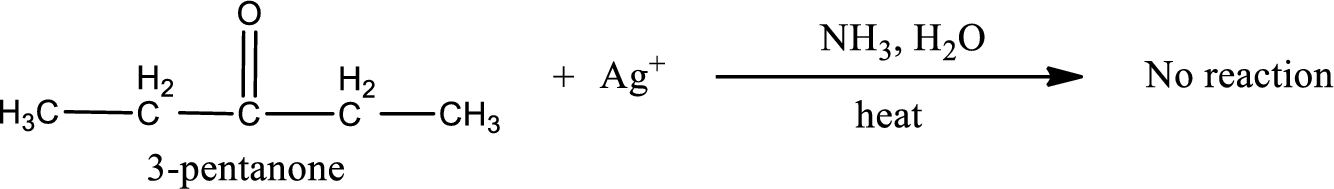
No inorganic product is formed when 3-pentanone undergoes Tollen’s test.
No reaction takes place when 3-pentanone undergoes Tollen’s test.
(c)
Interpretation:
The inorganic product formed when methylpropanal undergoes Benedict’s test has to be given.
Concept Introduction:
In organic chemistry, oxidation reaction is referred to the number
In organic chemistry, reduction reaction is referred to the number
Alcohols undergo oxidation reaction and reduction reaction. This depends upon the number of hydrogen atoms that is bonded to the alpha carbon atom. Primary and secondary alcohol undergoes oxidation reaction while tertiary alcohol does not undergo oxidation reaction. Primary alcohols undergo oxidation to give aldehyde and carboxylic acid as product. Secondary alcohol undergoes oxidation to give ketone as the product.
Aldehyde undergoes oxidation to give carboxylic acid as the product while ketone does not undergo oxidation reaction.
Tollen’s test:
This is also known as silver mirror test. The reagent that is used in Tollen’s test is silver nitrate and ammonia in water. Aldehyde reacts with Tollen’s reagent, where the silver ion is reduced to silver metal and the aldehyde is oxidized to carboxylic acid.
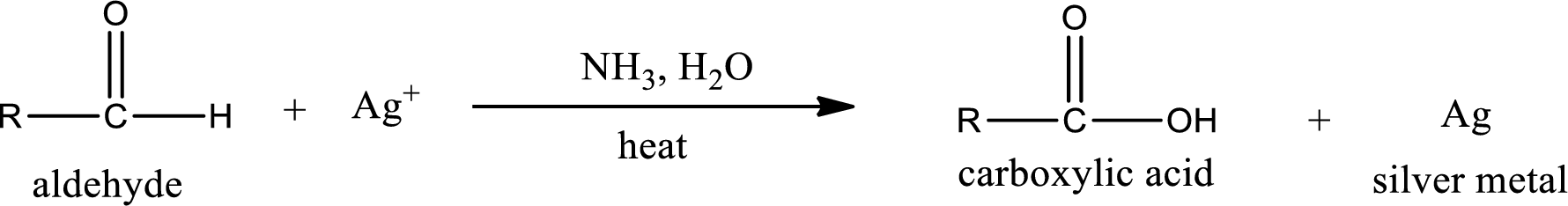
Ketone does not undergo Tollen’s test to deposit silver metal.
Benedict’s test:
This test is also similar to Tollen’s test. In this test,
Answer to Problem 15.80EP
The inorganic product formed is
Explanation of Solution
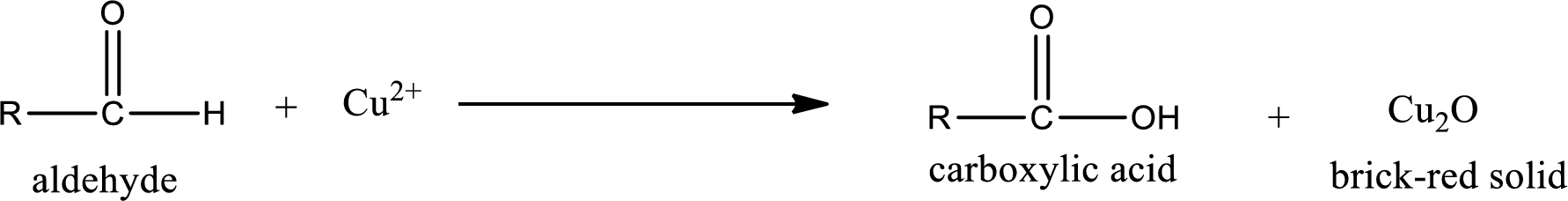
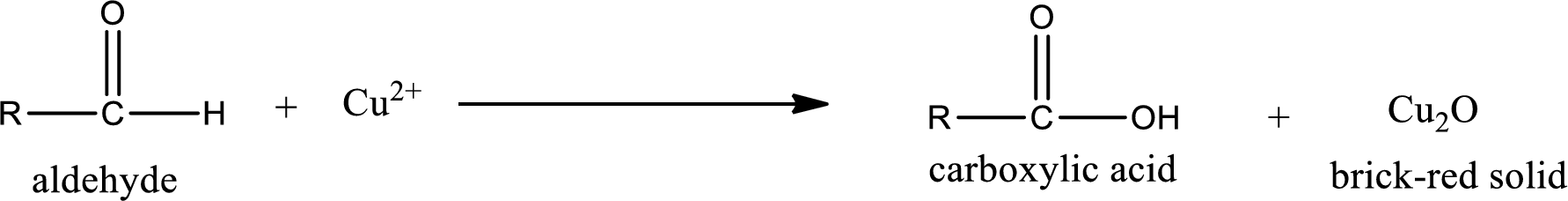
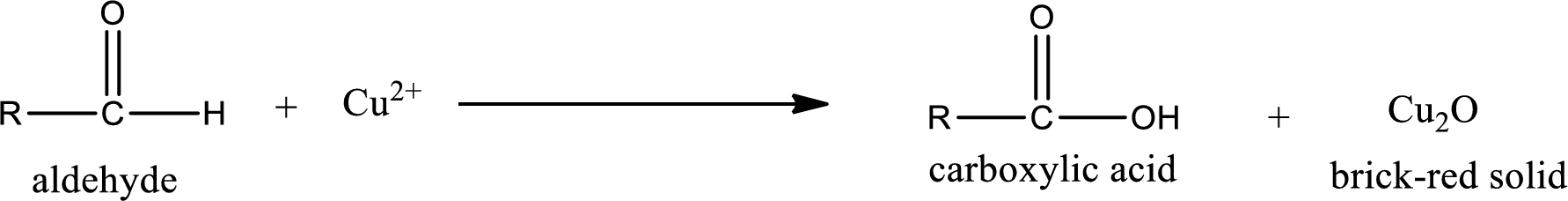
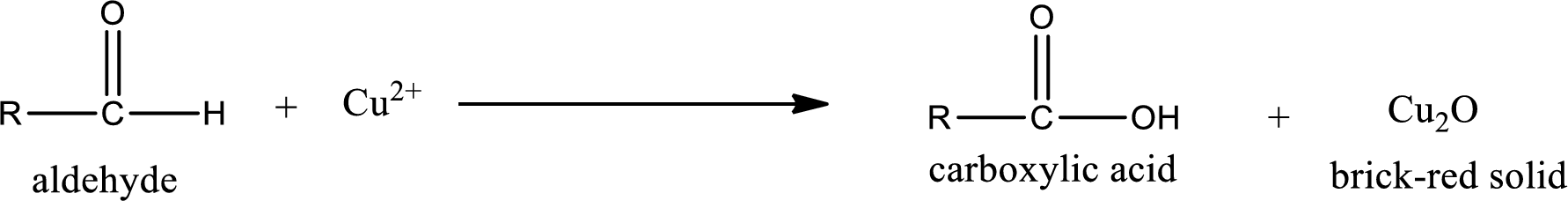
Aldehydes undergo Benedicts’s test. The product formed when aldehyde undergo oxidation is a carboxylic acid. The general oxidation reaction for aldehyde can be given as,
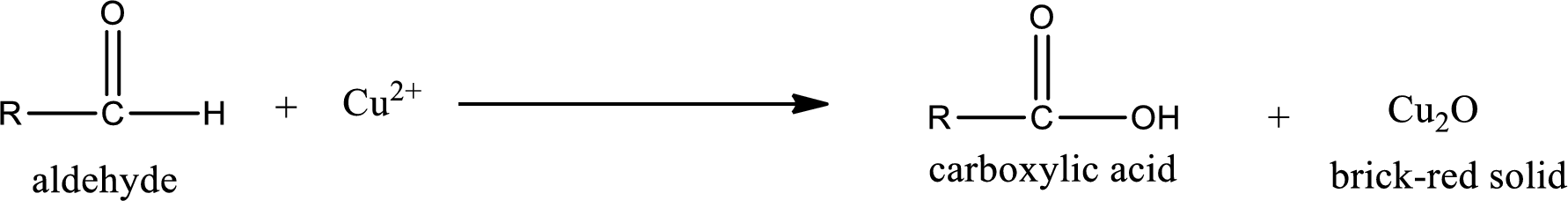
Given aldehyde is methylpropanal and the structure can be given as shown below,
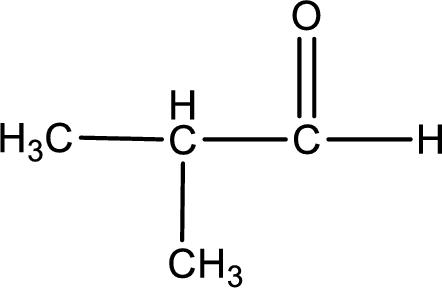
This on reaction with Tollen’s reagent gives carboxylic acid and Copper(I) oxide as the product. The inorganic product formed and the complete reaction can be given as shown below,
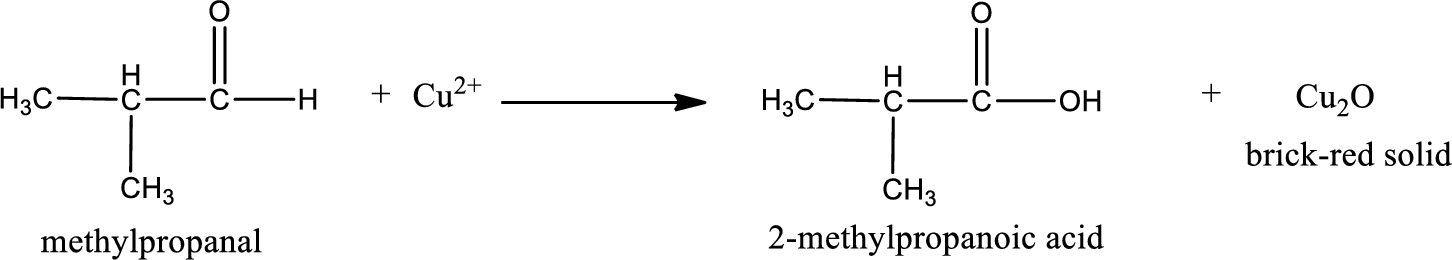
The inorganic product formed when methylpropanal undergoes Benedict’s test is given.
(d)
Interpretation:
The inorganic product formed when propanone undergoes Benedict’s test has to be given.
Concept Introduction:
In organic chemistry, oxidation reaction is referred to the number
In organic chemistry, reduction reaction is referred to the number
Alcohols undergo oxidation reaction and reduction reaction. This depends upon the number of hydrogen atoms that is bonded to the alpha carbon atom. Primary and secondary alcohol undergoes oxidation reaction while tertiary alcohol does not undergo oxidation reaction. Primary alcohols undergo oxidation to give aldehyde and carboxylic acid as product. Secondary alcohol undergoes oxidation to give ketone as the product.
Aldehyde undergoes oxidation to give carboxylic acid as the product while ketone does not undergo oxidation reaction.
Tollen’s test:
This is also known as silver mirror test. The reagent that is used in Tollen’s test is silver nitrate and ammonia in water. Aldehyde reacts with Tollen’s reagent, where the silver ion is reduced to silver metal and the aldehyde is oxidized to carboxylic acid.
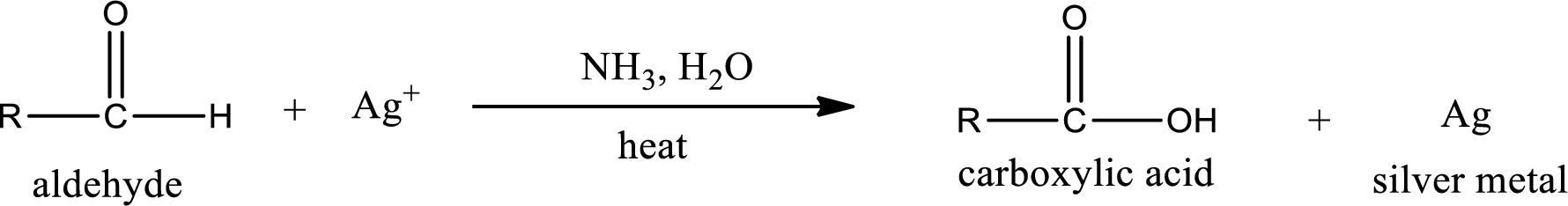
Ketone does not undergo Tollen’s test to deposit silver metal.
Benedict’s test:
This test is also similar to Tollen’s test. In this test,
Answer to Problem 15.80EP
No inorganic product is formed when propanone undergoes Benedict’s test.
Explanation of Solution
Aldehydes undergo Benedict’s test. The product formed when aldehyde undergo oxidation is a carboxylic acid. The general oxidation reaction for aldehyde can be given as,
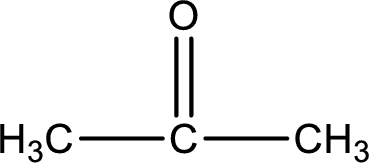
Given compound is a ketone. The name of ketone is propanone and the structure can be given as shown below,
This on reaction with Benedict’s reagent does not give oxidized product. Therefore, no reaction takes place when propanone undergoes Benedict’s test.
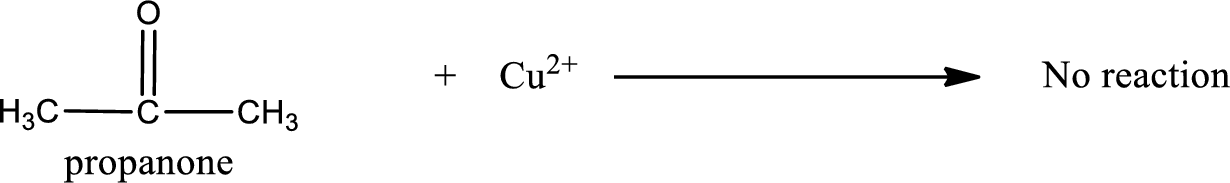
No inorganic product is formed when propanone undergo Benedict’s test.
No reaction takes place when propanone undergoes Benedict’s test.
Want to see more full solutions like this?
Chapter 15 Solutions
General, Organic, and Biological Chemistry
- Which of the following is the process that is "capable of destroying all forms of microbial life"? Question 37 options: Surgical scrub Sterilization Chemical removal Mechanical removalarrow_forwardAfter you feel comfortable with your counting method and identifying cells in the various stages of mitosis, use the four images below of whitefish blastula to count the cells in each stage until you reach 100 total cells, recording your data below in Data Table 1. (You may not need to use all four images. Stop counting when you reach 100 total cells.) After totaling the cells in each stage, calculate the percent of cells in each stage. (Divide total of stage by overall total of 100 and then multiply by 100 to obtain percentage.) Data Table 1Stage Totals PercentInterphase Mitosis: Prophase Metaphase Anaphase Telophase Cytokinesis Totals 100 100% To find the length of time whitefish blastula cells spend in each stage, multiply the percent (recorded as a decimal, in other words take the percent number and divide by 100) by 24 hours. (Example: If percent is 20%, then Time in Hours = .2 * 24 = 4.8) Record your data in Data…arrow_forwardWhat are Clathrin coated vesicles and what is their function?arrow_forward
- How is a protein destined for the Endoplasmic Reticulum (ER), imported into the ER? Be concise.arrow_forwardFind out about the organisations and the movements aimed at the conservation of our natural resources. Eg Chipko movement and Greenpeace. Make a project report on such an organisation.arrow_forwardWhat are biofertilizers and mention the significancearrow_forward
- PCBs and River Otters: Otters in Washington State’s Green-Duwamish River have high levels of polychlorinated biphenyls (PCBs) in their livers. PCBs can bind to the estrogen receptors in animals and disrupt the endocrine system of these otters. The PCBs seem to increase the estrogen to androgen ratio, skewing the ratio toward too much estrogen. How would increased estrogen affect the river otter population? Based on your reading of the materials in this unit, what factors can affect fertility in humans? Explain how each of the factors affecting human fertility that you described can disrupt the human endocrine system to affect reproduction.arrow_forwardOther than oil and alcohol, are there other liquids you could compare to water (that are liquid at room temperature)? How is water unique compared to these other liquids? What follow-up experiment would you like to do, and how would you relate it to your life?arrow_forwardSelection of Traits What adaptations do scavengers have for locating and feeding on prey? What adaptations do predators have for capturing and consuming prey?arrow_forward
- Competition Between Species What natural processes limit populations from growing too large? What are some resources organisms can compete over in their natural habitat?arrow_forwardSpecies Interactions Explain how predators, prey and scavengers interact. Explain whether predators and scavengers are necessary or beneficial for an ecosystem.arrow_forwardmagine that you are conducting research on fruit type and seed dispersal. You submitted a paper to a peer-reviewed journal that addresses the factors that impact fruit type and seed dispersal mechanisms in plants of Central America. The editor of the journal communicates that your paper may be published if you make ‘minor revisions’ to the document. Describe two characteristics that you would expect in seeds that are dispersed by the wind. Contrast this with what you would expect for seeds that are gathered, buried or eaten by animals, and explain why they are different. (Editor’s note: Providing this information in your discussion will help readers to consider the significance of the research).arrow_forward
 Comprehensive Medical Assisting: Administrative a...NursingISBN:9781305964792Author:Wilburta Q. Lindh, Carol D. Tamparo, Barbara M. Dahl, Julie Morris, Cindy CorreaPublisher:Cengage Learning
Comprehensive Medical Assisting: Administrative a...NursingISBN:9781305964792Author:Wilburta Q. Lindh, Carol D. Tamparo, Barbara M. Dahl, Julie Morris, Cindy CorreaPublisher:Cengage Learning Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning
Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning





