
(a)
Interpretation:
Structural formula for the given
Concept Introduction:
Structure of the ketone can be drawn from the IUPAC name. In the IUPAC name, the parent chain of carbon atom can be identified and then the substituents present in it can also be identified. With these information, the structure for the given compound can be drawn. In a ketone the counting has to be done so that the carbonyl carbon atom gets the least numbering.
The structural representation of organic compound can be done in 2D and 3D. In two-dimensional representation, there are four types of representation in which an organic compound can be drawn. They are,
- • Expanded structural formula
- • Condensed structural formula
- • Skeletal structural formula
- • Line-angle structural formula
Structural formula which shows all the atoms in a molecule along with all the bonds that is connecting the atoms present in the molecule is known as Expanded structural formula.
Structural formula in which grouping of atoms are done and in which the central atoms along with the other atoms are connected to them are treated as group is known as Condensed structural formula.
Structural formula that shows the bonding between carbon atoms alone in the molecule ignoring the hydrogen atoms being shown explicitly is known as Skeletal structural formula.
Structural formula where a line represent carbon‑carbon bond and the carbon atom is considered to be present in each point and the end of lines is known as Line-angle structural formula.
(a)
Answer to Problem 15.38EP
The structural formula for 2-methyl-3-pentanone is,
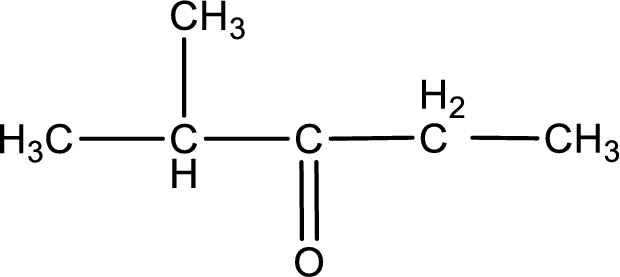
Explanation of Solution
The given name of the compound is 2-methyl-3-pentanone. From the name it is understood that the parent carbon chain is pentane and it contains five carbon atoms. The parent chain can be drawn as shown below,

From the name of the given ketone, the substituents that are present can be identified. In this case, the substituent is a methyl group on second carbon atom. The carbonyl carbon atom is the third carbon atom.
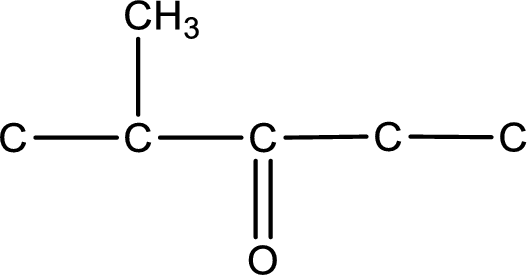
Carbon atom has a valence of four. Hence, carbon atom can form four covalent bonds. The remaining bonds are satisfied by hydrogen atom. The structure is obtained as shown below,
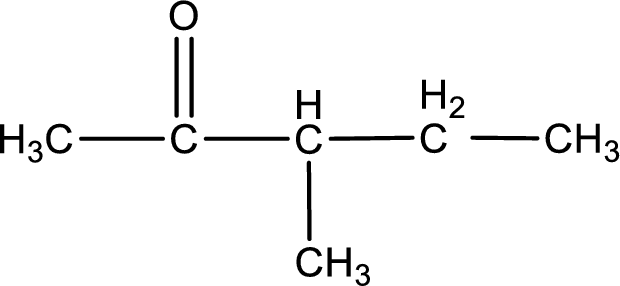
Structural formula for the given ketone is drawn.
(b)
Interpretation:
Structural formula for the given ketone has to be drawn.
Concept Introduction:
Structure of the ketone can be drawn from the IUPAC name. In the IUPAC name, the parent chain of carbon atom can be identified and then the substituents present in it can also be identified. With these information, the structure for the given compound can be drawn. In a ketone the counting has to be done so that the carbonyl carbon atom gets the least numbering.
The structural representation of organic compound can be done in 2D and 3D. In two-dimensional representation, there are four types of representation in which an organic compound can be drawn. They are,
- • Expanded structural formula
- • Condensed structural formula
- • Skeletal structural formula
- • Line-angle structural formula
Structural formula which shows all the atoms in a molecule along with all the bonds that is connecting the atoms present in the molecule is known as Expanded structural formula.
Structural formula in which grouping of atoms are done and in which the central atoms along with the other atoms are connected to them are treated as group is known as Condensed structural formula.
Structural formula that shows the bonding between carbon atoms alone in the molecule ignoring the hydrogen atoms being shown explicitly is known as Skeletal structural formula.
Structural formula where a line represent carbon‑carbon bond and the carbon atom is considered to be present in each point and the end of lines is known as Line-angle structural formula.
(b)
Answer to Problem 15.38EP
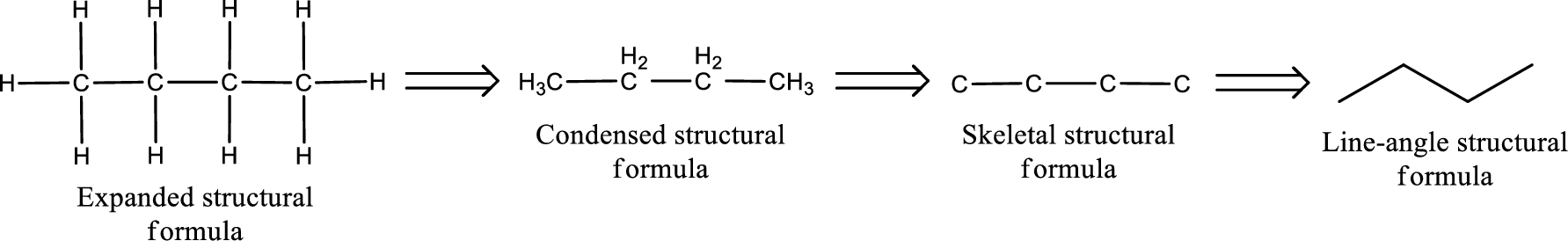
The structural formula for 2-pentanone is,
Explanation of Solution
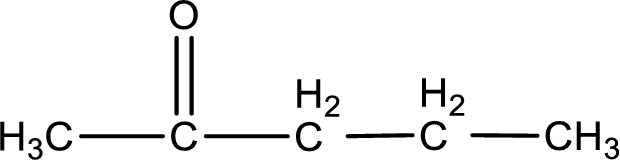
The given name of the compound is 2-pentanone. From the name it is understood that the parent carbon chain is pentane and it contains five carbon atoms. The parent chain can be drawn as shown below,

From the name of the given ketone, the substituents that are present can be identified. In this case, there are no substituents. The carbonyl carbon atom is the second carbon atom..
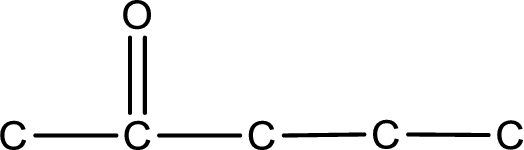
Carbon atom has a valence of four. Hence, carbon atom can form four covalent bonds. The remaining bonds are satisfied by hydrogen atom. The structure is obtained as shown below,
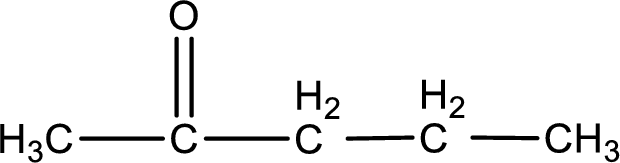
Structural formula for the given ketone is drawn.
(c)
Interpretation:
Structural formula for the given ketone has to be drawn.
Concept Introduction:
Structure of the ketone can be drawn from the IUPAC name. In the IUPAC name, the parent chain of carbon atom can be identified and then the substituents present in it can also be identified. With these information, the structure for the given compound can be drawn. In a ketone the counting has to be done so that the carbonyl carbon atom gets the least numbering.
The structural representation of organic compound can be done in 2D and 3D. In two-dimensional representation, there are four types of representation in which an organic compound can be drawn. They are,
- • Expanded structural formula
- • Condensed structural formula
- • Skeletal structural formula
- • Line-angle structural formula
Structural formula which shows all the atoms in a molecule along with all the bonds that is connecting the atoms present in the molecule is known as Expanded structural formula.
Structural formula in which grouping of atoms are done and in which the central atoms along with the other atoms are connected to them are treated as group is known as Condensed structural formula.
Structural formula that shows the bonding between carbon atoms alone in the molecule ignoring the hydrogen atoms being shown explicitly is known as Skeletal structural formula.
Structural formula where a line represent carbon‑carbon bond and the carbon atom is considered to be present in each point and the end of lines is known as Line-angle structural formula.
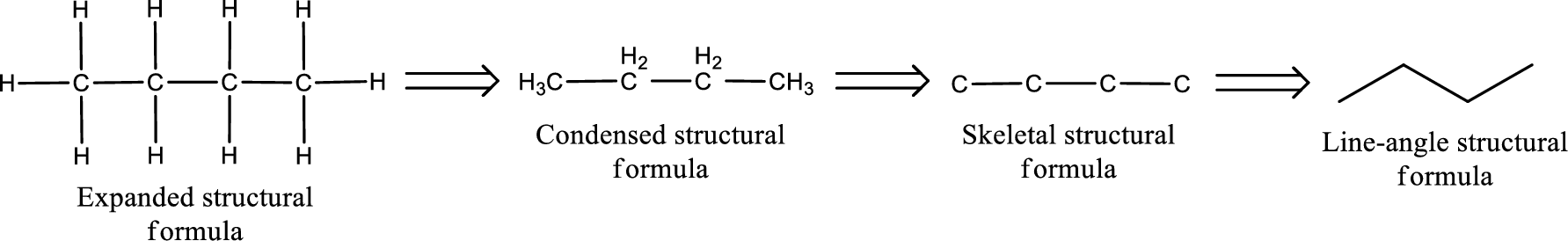
(c)
Answer to Problem 15.38EP
The structural formula for bromopropanone is,
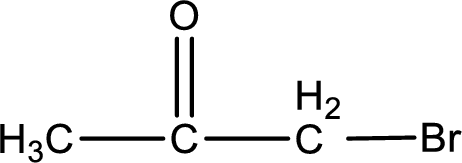
Explanation of Solution
The given name of the compound is bromopropanone. From the name it is understood that the parent carbon chain is propane and it contains three carbon atoms. The parent chain can be drawn as shown below,
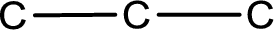
From the name of the given ketone, the substituents that are present can be identified. In this case a bromine atom is present as a substituent. The carbonyl carbon atom is the second carbon atom as that is the only possibility.
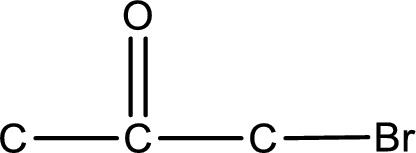
Carbon atom has a valence of four. Hence, carbon atom can form four covalent bonds. The remaining bonds are satisfied by hydrogen atom. The structure is obtained as shown below,
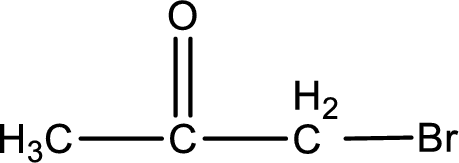
Structural formula for the given ketone is drawn.
(d)
Interpretation:
Structural formula for the given ketone has to be drawn.
Concept Introduction:
Structure of the ketone can be drawn from the IUPAC name. In the IUPAC name, the parent chain of carbon atom can be identified and then the substituents present in it can also be identified. With these information, the structure for the given compound can be drawn. In a ketone the counting has to be done so that the carbonyl carbon atom gets the least numbering.
The structural representation of organic compound can be done in 2D and 3D. In two-dimensional representation, there are four types of representation in which an organic compound can be drawn. They are,
- • Expanded structural formula
- • Condensed structural formula
- • Skeletal structural formula
- • Line-angle structural formula
Structural formula which shows all the atoms in a molecule along with all the bonds that is connecting the atoms present in the molecule is known as Expanded structural formula.
Structural formula in which grouping of atoms are done and in which the central atoms along with the other atoms are connected to them are treated as group is known as Condensed structural formula.
Structural formula that shows the bonding between carbon atoms alone in the molecule ignoring the hydrogen atoms being shown explicitly is known as Skeletal structural formula.
Structural formula where a line represent carbon‑carbon bond and the carbon atom is considered to be present in each point and the end of lines is known as Line-angle structural formula.
(d)
Answer to Problem 15.38EP
The structural formula for cyclopentanone is,
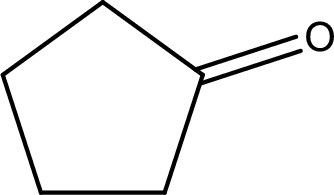
Explanation of Solution
The given name of the compound is cyclopentanone. From the name it is understood that the parent carbon chain is cyclic carbon chain that contains five carbon atoms. The parent chain can be drawn as shown below,
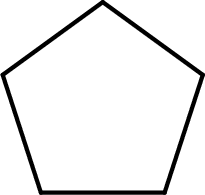
From the name of the given ketone, the substituents that are present can be identified. In this case, there are no substituents. The carbonyl carbon atom is any one of the carbon atom. Therefore, the structural formula of cyclopentanone can be drawn as shown below,
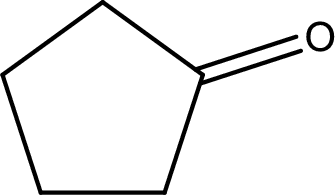
Structural formula for the given ketone is drawn.
Want to see more full solutions like this?
Chapter 15 Solutions
General, Organic, and Biological Chemistry
- Please help me solve this reaction.arrow_forwardIndicate the products obtained by mixing 2,2-dimethylpropanal with acetaldehyde and sodium ethoxide in ethanol.arrow_forwardSynthesize 2-Ethyl-3-methyloxirane from dimethyl(propyl)sulfonium iodide using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forward
- Synthesize 2-Hydroxy-2-phenylacetonitrile from phenylmethanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forward
- If possible, please provide the formula of the compound 3,3-dimethylbut-2-enal.arrow_forwardSynthesize 1,4-dibromobenzene from acetanilide (N-phenylacetamide) using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardIndicate the products obtained by mixing (3-oxo-3-phenylpropyl)triphenylphosphonium bromide with sodium hydride.arrow_forward
- We mix N-ethyl-2-hexanamine with excess methyl iodide and followed by heating with aqueous Ag2O. Indicate the major products obtained.arrow_forwardIndicate the products obtained by mixing acetophenone with iodine and NaOH.arrow_forwardIndicate the products obtained by mixing 2-Propanone and ethyllithium and performing a subsequent acid hydrolysis.arrow_forward
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
