
Chemistry & Chemical Reactivity
10th Edition
ISBN: 9781337399074
Author: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 14, Problem 80GQ
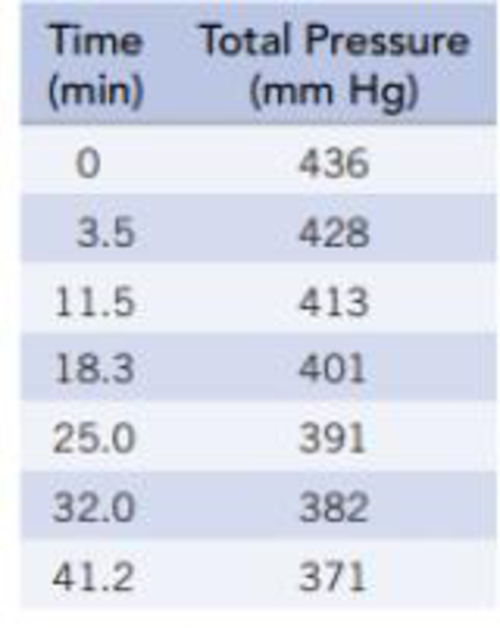
A The compound 1,3-butadiene (C4H6) forms 1,5-cyclooctadiene, C8H12 at higher temperatures.
C4H6(g) → ½ C8H12(g)
Use the following data to determine the order of the reaction and the rate constant, k. (Note that the total pressure is the pressure of the unreacted C4H6 at any time plus the pressure of the C8H12.)
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Draw a structure using wedges and dashes for the following compound:
H-
Et
OH
HO-
H
H-
Me
OH
Which of the following molecules are NOT typical carbohydrates? For the molecules that are
carbohydrates, label them as an aldose or ketose.
HO
Он
ОН ОН
Он
ОН
но
ΤΗ
HO
ОН
HO
eve
Он он
ОН
ОН
ОН
If polyethylene has an average molecular weight of 25,000 g/mol, how many repeat units
are present?
Draw the a-anomer cyclized pyranose Haworth projection of the below hexose. Circle the
anomeric carbons. Number the carbons on the Fischer and Haworth projections. Assign R
and S for each chiral center.
HO
CHO
-H
HO
-H
H-
-OH
H
-OH
CH₂OH
Draw the ẞ-anomer cyclized furanose Haworth projection for the below hexose. Circle the
anomeric carbons. Number the carbons on the Fischer and Haworth projections.
HO
CHO
-H
H
-OH
HO
-H
H
-OH
CH₂OH
Chapter 14 Solutions
Chemistry & Chemical Reactivity
Ch. 14.1 - Sucrose decomposes to fructose and glucose in acid...Ch. 14.1 - What are the relative rates of appearance or...Ch. 14.3 - The initial rate ( [NO]/ t] of the reaction of...Ch. 14.3 - The rate constant, k, at 25 C is 0.27/h for the...Ch. 14.4 - Sucrose, a sugar, decomposes in acid solution to...Ch. 14.4 - Gaseous azomethane (CH3N2CH3) decomposes to ethane...Ch. 14.4 - Prob. 14.7CYUCh. 14.4 - The catalyzed decomposition of hydrogen peroxide...Ch. 14.4 - Americium is used in smoke detectors and in...Ch. 14.5 - Prob. 14.10CYU
Ch. 14.5 - The colorless gas N2O4, decomposes to the brown...Ch. 14.7 - Nitrogen monoxide is reduced by hydrogen to give...Ch. 14.7 - Prob. 14.13CYUCh. 14.7 - One possible mechanism for the decomposition of...Ch. 14.7 - Prob. 1.1ACPCh. 14.7 - Prob. 1.2ACPCh. 14.7 - Prob. 2.1ACPCh. 14.7 - Prob. 2.2ACPCh. 14.7 - Prob. 2.3ACPCh. 14.7 - Determine the activation energy for the reaction...Ch. 14 - Give the relative rates of disappearance of...Ch. 14 - Give the relative rates of disappearance of...Ch. 14 - In the reaction 2 O3(g) 3 O2(g), the rate of...Ch. 14 - In the synthesis of ammonia, if [H2]/t = 4.5 104...Ch. 14 - Experimental data are listed here for the reaction...Ch. 14 - Phenyl acetate, an ester, reacts with water...Ch. 14 - Using the rate equation Rate = k[A]2[B], define...Ch. 14 - A reaction has the experimental rate equation Rate...Ch. 14 - The reaction between ozone and nitrogen dioxide at...Ch. 14 - Nitrosyl bromide, NOBr, is formed from NO and Br2:...Ch. 14 - The data in the table are for the reaction of NO...Ch. 14 - The reaction 2 NO(g) + 2 H2(g) N2(g) + 2 H2O(g)...Ch. 14 - Data for the reaction NO(g) + O2(g) NO2(g) are...Ch. 14 - Data for the following reaction are given in the...Ch. 14 - The rate equation for the hydrolysis of sucrose to...Ch. 14 - The decomposition of N2O5 in CCl4 is a first-order...Ch. 14 - The decomposition of SO2Cl2 is a first-order...Ch. 14 - The conversion of cyclopropane to propene (Example...Ch. 14 - Hydrogen peroxide, H2O2(aq), decomposes to H2O()...Ch. 14 - The decomposition of nitrogen dioxide at a high...Ch. 14 - At 573 K, gaseous NO2(g) decomposes, forming NO(g)...Ch. 14 - The dimerization of butadiene, C4H6, to form...Ch. 14 - The decomposition of ammonia on a metal surface to...Ch. 14 - Hydrogen iodide decomposes when heated, forming...Ch. 14 - The rate equation for the decomposition of N2O5...Ch. 14 - Gaseous azomethane, CH3N=NCH3, decomposes in a...Ch. 14 - The decomposition of SO2Cl2 SO2Cl2(g) SO2(g) +...Ch. 14 - The compound Xe(CF3)2 decomposes in a first-order...Ch. 14 - The radioactive isotope 64Cu is used in the form...Ch. 14 - Radioactive gold-198 is used in the diagnosis of...Ch. 14 - Prob. 31PSCh. 14 - Ammonia decomposes when heated according to the...Ch. 14 - Gaseous NO2 decomposes at 573 K. NO2(g) NO(g) + ...Ch. 14 - The decomposition of HOF occurs at 25 C. HOF(g) ...Ch. 14 - Prob. 35PSCh. 14 - Prob. 36PSCh. 14 - Calculate the activation energy, Ea, for the...Ch. 14 - If the rate constant for a reaction triples when...Ch. 14 - When healed lo a high temperature, cyclobutane,...Ch. 14 - When heated, cyclopropane is converted to propene...Ch. 14 - The reaction of H2 molecules with F atoms H2(g) +...Ch. 14 - Prob. 42PSCh. 14 - Compare the lock-and-key and induced-fit models...Ch. 14 - Prob. 44PSCh. 14 - Prob. 45PSCh. 14 - The enzyme carbonic anhydrase catalyzes the...Ch. 14 - What is the rate law for each of the following...Ch. 14 - What is the rate law for each of the following...Ch. 14 - Ozone, O3, in the Earths upper atmosphere...Ch. 14 - The reaction of NO2(g) and CO(g) is thought to...Ch. 14 - A proposed mechanism for the reaction of NO2 and...Ch. 14 - The mechanism for the reaction of CH3OH and HBr is...Ch. 14 - A reaction has the following experimental rate...Ch. 14 - For a first-order reaction, what fraction of...Ch. 14 - Prob. 55GQCh. 14 - Data for the following reaction are given in the...Ch. 14 - Formic acid decomposes at 550 C according to the...Ch. 14 - Isomerization of CH3NC occurs slowly when CH3NC is...Ch. 14 - When heated, tetrafluoroethylene dimerizes to form...Ch. 14 - Data in the table were collected at 540 K for the...Ch. 14 - Ammonium cyanate, NH4NCO, rearranges in water to...Ch. 14 - Prob. 62GQCh. 14 - At temperatures below 500 K, the reaction between...Ch. 14 - Nitryl fluoride can be made by treating nitrogen...Ch. 14 - The decomposition of dinitrogen pentaoxide N2O5(g)...Ch. 14 - The data in the table give the temperature...Ch. 14 - The decomposition of gaseous dimethyl ether at...Ch. 14 - The decomposition of phosphine, PH3, proceeds...Ch. 14 - The thermal decomposition of diacetylene, C4H2,...Ch. 14 - Prob. 70GQCh. 14 - The ozone in the Earths ozone layer decomposes...Ch. 14 - Hundreds of different reactions occur in the...Ch. 14 - Data for the reaction [Mn(CO)5(CH3CN)]+ + NC5H5 ...Ch. 14 - The gas-phase reaction 2 N2O5(g) 4 NO2(g) + O2(g)...Ch. 14 - Prob. 75GQCh. 14 - The decomposition of SO2Cl2 to SO2 and Cl2 is...Ch. 14 - The decomposition of nitrogen dioxide at a high...Ch. 14 - Prob. 78GQCh. 14 - Egg protein albumin is precipitated when an egg is...Ch. 14 - A The compound 1,3-butadiene (C4H6) forms...Ch. 14 - Hypofluorous acid, HOF, is very unstable,...Ch. 14 - We know that the decomposition of SO2Cl2 is...Ch. 14 - Nitramide, NO2NH2, decomposes slowly in aqueous...Ch. 14 - Prob. 84GQCh. 14 - The color change accompanying the reaction of...Ch. 14 - Prob. 87ILCh. 14 - Prob. 88ILCh. 14 - The oxidation of iodide ion by the hypochlorite...Ch. 14 - The acid-catalyzed iodination of acetone...Ch. 14 - Prob. 91SCQCh. 14 - The following statements relate to the reaction...Ch. 14 - Chlorine atoms contribute to the destruction of...Ch. 14 - Prob. 95SCQCh. 14 - Prob. 96SCQCh. 14 - The reaction cyclopropane propene occurs on a...Ch. 14 - Prob. 98SCQCh. 14 - Examine the reaction coordinate diagram given...Ch. 14 - Draw a reaction coordinate diagram for an...Ch. 14 - Consider the reaction of ozone and nitrogen...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Label each statement about the polynucleotide ATGGCG as true or false. The polynucleotide has six nucleotides. ...
General, Organic, and Biological Chemistry - 4th edition
Separate the list P,F,V,,T,a,m,L,t, and V into intensive properties, extensive properties, and nonproperties.
Fundamentals Of Thermodynamics
45. Calculate the mass of nitrogen dissolved at room temperature in an 80.0-L home aquarium. Assume a total pre...
Chemistry: Structure and Properties (2nd Edition)
Why is it unlikely that two neighboring water molecules would be arranged like this?
Campbell Biology (11th Edition)
Give the IUPAC name for each compound.
Organic Chemistry
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Name the below disaccharide. Circle any hemiacetals. Identify the numbering of glycosidic linkage, and identify it as a or ẞ. OH HO HO OH HO HO HO OHarrow_forwardWhat are the monomers used to make the following polymers? F. а. b. с. d. Вецер хочому なarrow_forward1. Propose a reasonable mechanism for the following transformation. I'm looking for curved mechanistic arrows and appropriate formal charges on intermediates. OMe MeO OMe Me2N NMe2 OTBS OH xylenes OMe 'OTBSarrow_forward
- What is the polymer made from the following monomers? What type of polymerization is used for each? а. ОН H2N но b. ن -NH2 d. H₂N NH2 довarrow_forwardCondensation polymers are produced when monomers containing two different functional groups link together with the loss of a small molecule such as H2O. The difunctional monomer H2N(CH2)6COOH forms a condensation polymer. Draw the carbon-skeleton structure of the dimer that forms from this monomer.arrow_forwardWhat is the structure of the monomer?arrow_forward
- → BINDERIYA GANBO... BINDERIYA GANBO. AP Biology Notes Gamino acid chart - G... 36:22 司 10 ☐ Mark for Review Q 1 Hide 80 8 2 =HA O=A¯ = H₂O Acid HIO HBrO HCIO Question 10 of 35 ^ Σ DELL □ 3 % Λ & 6 7 * ∞ 8 do 5 $ 4 # m 3 ° ( 9 Highlights & Notes AXC Sign out Carrow_forwardWhich representation(s) show polymer structures that are likely to result in rigid, hard materials and those that are likely to result in flexible, stretchable, soft materials?arrow_forward3. Enter the molecular weight of the product obtained from the Williamson Ether Synthesis? OH OH & OH excess CH3l Ag₂Oarrow_forward
- Please answer 1, 2 and 3 on the endarrow_forwardIn the box below, specify which of the given compounds are very soluble in polar aprotic solvents. You may select more than one compound. Choose one or more: NaCl NH4Cl CH3CH2CH2CH2CH2CN CH3CH2OH hexan-2-one NaOH CH3SCH3arrow_forwardOn the following structure, select all of the atoms that could ACCEPT a hydrogen bond. Ignore possible complications of aromaticity. When selecting be sure to click on the center of the atom.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning
Kinetics: Chemistry's Demolition Derby - Crash Course Chemistry #32; Author: Crash Course;https://www.youtube.com/watch?v=7qOFtL3VEBc;License: Standard YouTube License, CC-BY