
EBK FUNDAMENTALS OF GENERAL, ORGANIC, A
8th Edition
ISBN: 8220102895805
Author: Peterson
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Question
Chapter 14, Problem 14.43AP
Interpretation Introduction
Interpretation:
The formation of product should be identified when the alcohol reaction with sodium metal.
Concept Introduction:
Alcohol reaction with sodium:
Any alcohol undergoes reaction with sodium (
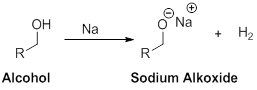
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
To map the active site of beta-lactamase, the enzyme was hydrolyzed with trypsin to yield a hexapeptide (P1) with the following amino acids. Glu, Lys, Leu, Phe, Met, and Ser. Treatment of P1 with phenyl isothiocyanate yielded a PTH derivative of phenylalanine and a peptide (P2). Treatment of P1 with cyanogenbromide gave an acidic tetrapeptide (P3) and a dipeptide (P4).Treatment of P2 with 1-fluoro-2,4-dinitrobenzene, followed by complete hydrolysis, yields N-2,4-dinitrophenyl-Glu. P1, P2, and P3 contain the active site serine.
Why doesn't D in this hexapeptide not participate in the hydrolysis of the beta-lactam ring even though S, K, and D are involved in the catalyst?
To map the active site of -lactamase, the enzyme was hydrolyzed with trypsin to yield a hexapeptide (P1) with the following amino acids. Glu, Lys, Leu, Phe, Met, and Ser. Treatment of P1 with phenyl isothiocyanate yielded a PTH derivative of phenylalanine and a peptide (P2). Treatment of P1 with cyanogenbromide gave an acidic tetrapeptide (P3) and a dipeptide (P4).Treatment of P2 with 1-fluoro-2,4-dinitrobenzene, followed by complete hydrolysis, yields N-2,4-dinitrophenyl-Glu. P1, P2, and P3 contain the active site serine.
Using the experimental results described above derive the primary sequence of the active site hexapeptide. Please help!
Which type of enzyme catalyses the following reaction? oxidoreductase, transferase, hydrolase, lyase, isomerase, or ligase.
Chapter 14 Solutions
EBK FUNDAMENTALS OF GENERAL, ORGANIC, A
Ch. 14.1 - Identify each of the following compounds as an...Ch. 14.1 - Prob. 14.2PCh. 14.2 - Prob. 14.3PCh. 14.2 - Prob. 14.4PCh. 14.2 - Prob. 14.5PCh. 14.3 - Prob. 14.6PCh. 14.3 - For each of the following molecules, (i) redraw...Ch. 14.4 - Prob. 14.1MRPCh. 14.4 - Provide the mechanism for the dehydration of...Ch. 14.4 - Prob. 14.3MRP
Ch. 14.4 - Prob. 14.8PCh. 14.4 - What alcohols yield the following alkenes as the...Ch. 14.4 - Prob. 14.10KCPCh. 14.4 - What products would you expect from oxidation of...Ch. 14.4 - Prob. 14.12PCh. 14.4 - Prob. 14.13KCPCh. 14.5 - Prob. 14.14PCh. 14.5 - Prob. 14.15PCh. 14.7 - Prob. 14.1CIAPCh. 14.7 - Prob. 14.2CIAPCh. 14.7 - Prob. 14.3CIAPCh. 14.7 - Prob. 14.16PCh. 14.8 - What disulfides would you obtain from oxidation of...Ch. 14.9 - Prob. 14.18PCh. 14.10 - Prob. 14.19PCh. 14.10 - Prob. 14.20PCh. 14.10 - Prob. 14.4CIAPCh. 14.10 - Prob. 14.5CIAPCh. 14.10 - Prob. 14.6CIAPCh. 14.10 - Prob. 14.7CIAPCh. 14 - Prob. 14.21UKCCh. 14 - Prob. 14.22UKCCh. 14 - Prob. 14.23UKCCh. 14 - Prob. 14.24UKCCh. 14 - Prob. 14.25UKCCh. 14 - How do alcohols, ethers, and phenols differ...Ch. 14 - What is the structural difference between primary,...Ch. 14 - Prob. 14.28APCh. 14 - Prob. 14.29APCh. 14 - The Taxane nucleus is shown here; it is the basis...Ch. 14 - Vitamin E has the structure shown. Identify the...Ch. 14 - Give systematic names for the following alcohols:...Ch. 14 - Give systematic names for the following compound...Ch. 14 - Draw structures corresponding to the following...Ch. 14 - Draw structures corresponding to the following...Ch. 14 - Prob. 14.36APCh. 14 - Locate the alcohol functional groups in the taxane...Ch. 14 - Prob. 14.38APCh. 14 - Prob. 14.39APCh. 14 - Prob. 14.40APCh. 14 - Prob. 14.41APCh. 14 - Prob. 14.42APCh. 14 - Prob. 14.43APCh. 14 - Assume that you have samples of the following two...Ch. 14 - Which of the following alcohols can undergo...Ch. 14 - The following alkenes can be prepared by...Ch. 14 - Prob. 14.47APCh. 14 - Prob. 14.48APCh. 14 - What alcohols would you oxidize to obtain the...Ch. 14 - Prob. 14.50APCh. 14 - What is the structural relationship between a...Ch. 14 - Prob. 14.52APCh. 14 - Prob. 14.53APCh. 14 - Prob. 14.54APCh. 14 - Prob. 14.55APCh. 14 - Prob. 14.56APCh. 14 - Prob. 14.57APCh. 14 - Identify the chiral center(s) in each of the...Ch. 14 - Are the following molecules chiral or achiral? If...Ch. 14 - Prob. 14.60CPCh. 14 - Prob. 14.61CPCh. 14 - 1-Propanol is freely soluble in water, 1-butanol...Ch. 14 - Prob. 14.63CPCh. 14 - Prob. 14.64CPCh. 14 - Prob. 14.65CPCh. 14 - Prob. 14.66CPCh. 14 - Prob. 14.67CPCh. 14 - Prob. 14.68CPCh. 14 - Prob. 14.69CPCh. 14 - Prob. 14.70CPCh. 14 - Prob. 14.71CPCh. 14 - Prob. 14.72CPCh. 14 - (a)Draw all possible cyclic C7H14O alcohol isomers...Ch. 14 - Prob. 14.74GPCh. 14 - Prob. 14.75GP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Similar questions
- +NH+ CO₂ +P H₂N + ATP H₂N NH₂ +ADParrow_forwardWhich type of enzyme catalyses the following reaction? oxidoreductase, transferase, hydrolase, lyase, isomerase, or ligase.arrow_forwardWhich features of the curves in Figure 30-2 indicates that the enzyme is not consumed in the overall reaction? ES is lower in energy that E + S and EP is lower in energy than E + P. What does this tell you about the stability of ES versus E + S and EP versus E + P.arrow_forward
- multiple choice urgent!arrow_forwardplease answer all questions 1 identify the amino acids below by name and three letter abbrevarrow_forwardPyruvate is accepted into the TCA cycle by a “feeder” reaction using the pyruvatedehydrogenase complex, resulting in acetyl-CoA and CO2. Provide a full mechanismfor this reaction utilizing the TPP cofactor. Include the roles of all cofactors.arrow_forward
- B- Vitamins are converted readily into important metabolic cofactors. Deficiency inany one of them has serious side effects. a. The disease beriberi results from a vitamin B 1 (Thiamine) deficiency and ischaracterized by cardiac and neurological symptoms. One key diagnostic forthis disease is an increased level of pyruvate and α-ketoglutarate in thebloodstream. How does this vitamin deficiency lead to increased serumlevels of these factors? b. What would you expect the effect on the TCA intermediates for a patientsuffering from vitamin B 5 deficiency? c. What would you expect the effect on the TCA intermediates for a patientsuffering from vitamin B 2 /B 3 deficiency?arrow_forwardDraw the Krebs Cycle and show the entry points for the amino acids Alanine,Glutamic Acid, Asparagine, and Valine into the Krebs Cycle - (Draw the Mechanism). How many rounds of Krebs will be required to waste all Carbons of Glutamic Acidas CO2?arrow_forwardSodium fluoroacetate (FCH 2CO2Na) is a very toxic molecule that is used as rodentpoison. It is converted enzymatically to fluoroacetyl-CoA and is utilized by citratesynthase to generate (2R,3S)-fluorocitrate. The release of this product is a potentinhibitor of the next enzyme in the TCA cycle. Show the mechanism for theproduction of fluorocitrate and explain how this molecule acts as a competitiveinhibitor. Predict the effect on the concentrations of TCA intermediates.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College
Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College Human Biology (MindTap Course List)BiologyISBN:9781305112100Author:Cecie Starr, Beverly McMillanPublisher:Cengage Learning
Human Biology (MindTap Course List)BiologyISBN:9781305112100Author:Cecie Starr, Beverly McMillanPublisher:Cengage Learning




Anatomy & Physiology
Biology
ISBN:9781938168130
Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark Womble
Publisher:OpenStax College


Human Biology (MindTap Course List)
Biology
ISBN:9781305112100
Author:Cecie Starr, Beverly McMillan
Publisher:Cengage Learning
GCSE Chemistry - Acids and Bases #34; Author: Cognito;https://www.youtube.com/watch?v=vt8fB3MFzLk;License: Standard youtube license