
Concept explainers
Interpretation:
Solid are the chemical substance which are characterised by definite shape and volume rigidity, high density, low compressibility. The constituent particles (atoms molecules or ions are closely packed and held together by strong force.
Types of solid:
The solid are of two types crystalline solids and amorphous solids.
Unit cell:
The smallest geometrical portion of the crystal lattice which can be as repetitive unit to build up the whole crystal is called unit cell.
Types of unit cell:
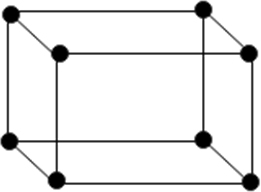
(i) Simple or primitive unit: In which the particle are present at the corners only.
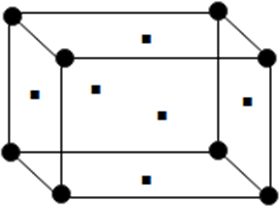
(ii) Face centred unit cell: In which the particle are present at the corners as well as at the centre of each of six faces.
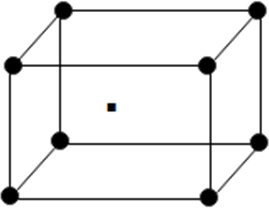
(iii) Body centred unit cell: In which the particles are present at the corners as well at the centre of unit.
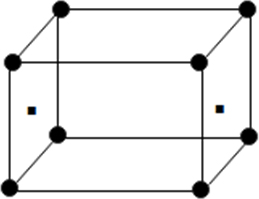
(iv) End centred unit cell: In which the particles are present at the corners and at the centre of two opposite faces.
Concept introduction:
No of particles and their contribution
| Unit cell | Corner | Face | Centre | Total |
| Body centred Unit cell |
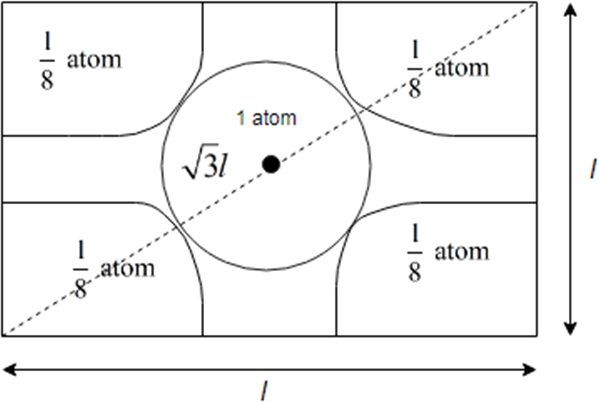
It is cross section view of body centered unit cell.
From above it becomes clear that body centred cubic unit cell atoms touch each other along the body diagonal
So length of body diagonal
To determine: the radius of the atom in pm in a body centered cubic unit cell that has volume of
Want to see the full answer?
Check out a sample textbook solution
Chapter 13 Solutions
Chemistry: Structure and Properties Custom Edition for Rutgers University General Chemistry
- You're competing on a Great British television game show, and you need to bake a cake. The quantity for each ingredient is given in grams, but you haven't been given a kitchen scale. Which of these properties would correlate with the mass of a baking ingredient like eggs or milk? Check all that apply. depth of color viscosity volume densityarrow_forwardDraw a Lewis structure for each of the following species. Again, assign charges where appropriate. a. H-H¯ b. CH3-CH3 c. CH3+CH3 d. CH3 CH3 e. CH3NH3+CH3NH3 f. CH30-CH3O¯ g. CH2CH2 - h. HC2-(HCC) HC2 (HCC) i. H202×(HOOH) H₂O₂ (HOOH) Nortonarrow_forwardIs molecule 6 an enantiomer?arrow_forward
- Show work. Don't give Ai generated solutionarrow_forwardCheck the box under each structure in the table that is an enantiomer of the molecule shown below. If none of them are, check the none of the above box under the table. Molecule 1 Molecule 2 Molecule 3 ----||| Molecule 4 Molecule 5 Molecule 6 none of the above mm..arrow_forwardShow work. don't give Ai generated solutionarrow_forward
- Check the box under each structure in the table that is an enantiomer of the molecule shown below. If none of them are, check the none of the above box under the table. Molecule 1 Molecule 2 Molecule 3 ----||| Molecule 4 Molecule 5 Molecule 6 none of the above mm..arrow_forwardUse the vapor-liquid equilibrium data at 1.0 atm. for methanol-water (Table 2-8 ) for the following: If the methanol vapor mole fraction is 0.600, what is the methanol liquid mole fraction? Is there an azeotrope in the methanol-water system at a pressure of 1.0 atmospheres? If water liquid mole fraction is 0.350, what is the water vapor mole fraction? What are the K values of methanol and of water at a methanol mole fraction in the liquid of 0.200? What is the relative volatility αM-W at a methanol mole fraction in the liquid of 0.200?arrow_forwardCheck the box under each structure in the table that is an enantiomer of the molecule shown below. If none of them are, check the none of the above box under the table. || |II***** Molecule 1 | Molecule 4 none of the above Molecule 2 Molecule 3 Х mm... C ---||| *** Molecule 5 Molecule 6arrow_forward
- is SiBr4 Silicon (IV) tetra Bromine? is KClO2 potassium dihypochlorite ?arrow_forward"יוון HO" Br CI Check the box under each structure in the table that is an enantiomer of the molecule shown below. If none of them are, check the none of the above box under the table. Molecule 1 Molecule 2 Molecule 3 Br Br Br HO OH H CI OH ✓ Molecule 4 Molecule 5 Molecule 6 CI Br יייון H Br OH OH CI Br ☐ none of the above × Garrow_forwardUS2 Would this be Uranium (II) diSulfide?arrow_forward
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning


