
Concept explainers
Interpretation:
Solid are the chemical substance which are characterised by definite shape and volume rigidity, high density, low compressibility. The constituent particles (atoms molecules or ions are closely packed and held together by strong force.
Types of solid:
The solid are of two types crystalline solids and amorphous solids.
Unit cell:
The smallest geometrical portion of the crystal lattice which can be as repetitive unit to build up the whole crystal is called unit cell.
Types of unit cell:
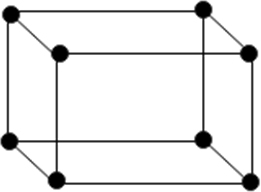
(i) Simple or primitive unit: In which the particle are present at the corners only.
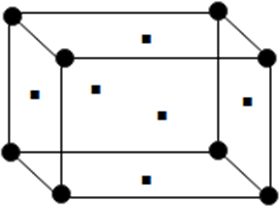
(ii) Face centred unit cell: In which the particle are present at the corners as well as at the centre of each of six faces.
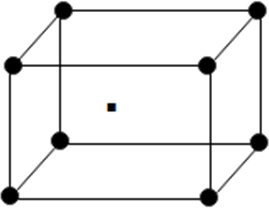
(iii) Body centred unit cell: In which the particles are present at the corners as well at the centre of unit.
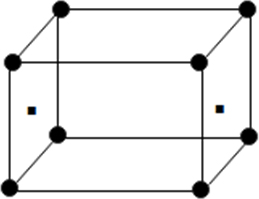
(iv) End centred unit cell: In which the particles are present at the corners and at the centre of two opposite faces.
Concept introduction:
No of particles and their contribution
| Unit cell | Corner | Face | Centre | Total |
| Body centred Unit cell |
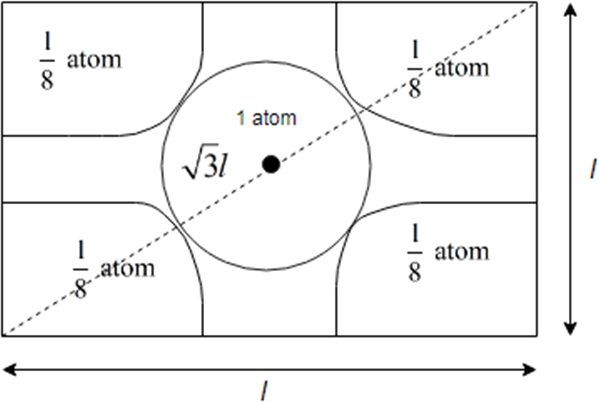
It is cross section view of body centered unit cell.
From above it becomes clear that body centred cubic unit cell atoms touch each other along the body diagonal
So length of body diagonal
To determine: the radius of the atom in pm in a body centered cubic unit cell that has volume of
Want to see the full answer?
Check out a sample textbook solution
Chapter 13 Solutions
Chemistry: Structure and Properties
- In reactions whose kinetic equation is v = k[A]m, the rate coefficient k is always positive. Is this correct?arrow_forwardIf the concentration of A decreases exponentially with time, what is the rate equation? (A). -d[A] (B). dt d[A] = k[A] e-kt dtarrow_forwardGiven the first-order reaction: aA → products. State its kinetic equation.arrow_forward
- The following chemical structure represents a molecule of what molecular formula?arrow_forwardWhich region(s) of the following phospholipid is/are hydrophobic? RO I hydro-water phobic-dislikes = Hydrophobic dislikes water ○ I only Il only I and III only II and IV only O II, III, and IV only III || IVarrow_forwardPredict the product of the following reactions: O 0= excess Х Кон ОН H+ H+ Iarrow_forward
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning


