
Interpretation:
Solid: Solid is a sample of matter that retains its shape and density when not confined. Stronger forces of attraction exist between atom/ions/ molecules.
Liquid: A liquid is a sample of matter that conforms to shape of container in which acquires a defined surface in the presence of gravity. In this state weaker force exist between atoms/ ions/ molecules.
Gas: A gas is a sample of matter that conforms to the shape of container in which it is placed and acquires whole volume of container.
Negligible forces of attraction exist between atoms/ions/molecules.
Supercritical fluid: Fluid over its critical temperature and pressure exhibiting good solvent power.
Boling point: The boiling point of a substance is the temperature at which the vapour pressure of the liquid equal the pressure surrounding the liquid and liquid change in vapour
Normal point: The normal boiling point of a substance is defined as the temperature at which the vapour pressure of the liquid becomes equal to at the sea level.
Concept introduction:
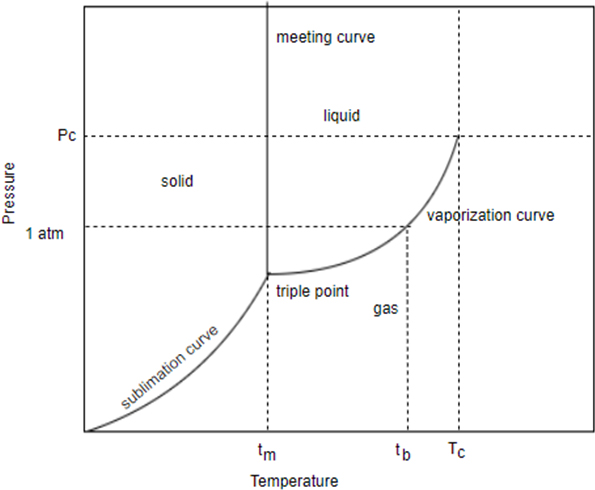
This is the generic phase diagram. This graph show phase change as a function of temperature and pressure.
To determine:
Nitrogen has normal Boiling point at and a melting point of its critical temperature is and its critical pressure is torr it has triple point at and torr sketch the phase diagram for nitrogen. Does nitrogen have a stable liquid state at
Want to see the full answer?
Check out a sample textbook solution
Chapter 13 Solutions
Chemistry: Structure and Properties
- If the concentration of A decreases exponentially with time, what is the rate equation? (A). -d[A] (B). dt d[A] = k[A] e-kt dtarrow_forwardGiven the first-order reaction: aA → products. State its kinetic equation.arrow_forwardDetermine the symmetry of the combination of atomic orbitals for bf 4-arrow_forward
- Which region(s) of the following phospholipid is/are hydrophobic? RO I hydro-water phobic-dislikes = Hydrophobic dislikes water ○ I only Il only I and III only II and IV only O II, III, and IV only III || IVarrow_forwardPredict the product of the following reactions: O 0= excess Х Кон ОН H+ H+ Iarrow_forwardHow many chiral centers/stereocenters are there in the following molecule? 1 2 3 4arrow_forward
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning





