
Chemistry: Structure and Properties
1st Edition
ISBN: 9780321834683
Author: Nivaldo J. Tro
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Question
Chapter 13, Problem 52E
Interpretation Introduction
Interpretation:
You have to determine which state is denser. The solid or liquid with explanation if you know triple point and melting point.
Concept introduction:
If the solid phase is less dense than the liquid phase, then the line separates solid and liquids bends left.
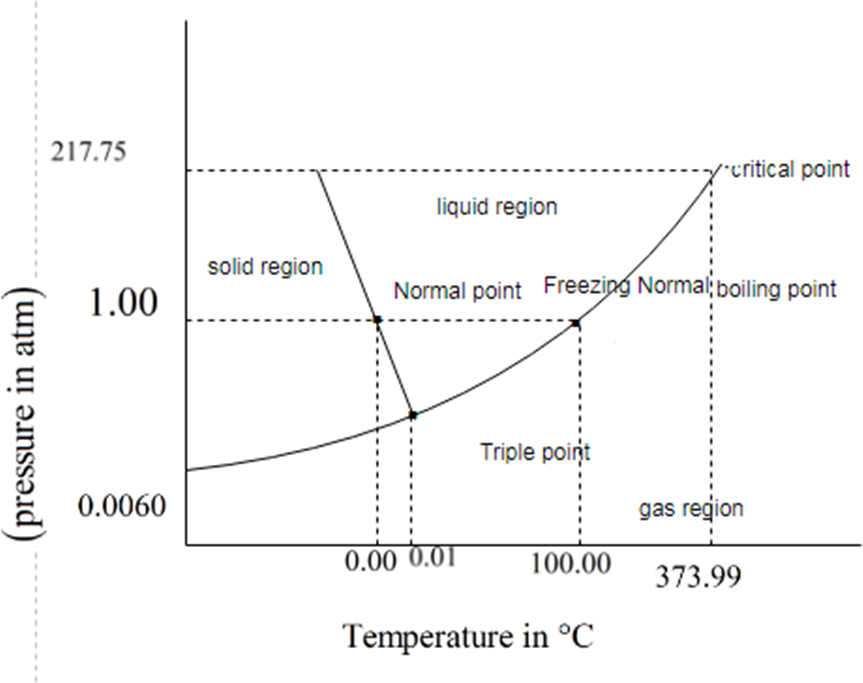
Melting curve is inclined to the solid phase is mole dense than the liquid phase. The line that separates solid and liquid bends right.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Michael Reactions
19.52 Draw the products from the following Michael addition reactions.
1.
H&C CH
(a)
i
2. H₂O*
(b)
OEt
(c)
EtO
H₂NEt
(d)
ΕΙΟ
+
1. NaOEt
2. H₂O'
H
H
1. NaOEt
2. H₂O*
Rank the labeled protons (Ha-Hd) in order of increasing acidity, starting with the least acidic.
НОН НЬ
OHd
Онс
Can the target compound at right be efficiently synthesized in good yield from the unsubstituted benzene at left?
?
starting
material
target
If so, draw a synthesis below. If no synthesis using reagents ALEKS recognizes is possible, check the box under the drawing area.
Be sure you follow the standard ALEKS rules for submitting syntheses.
+ More...
Note for advanced students: you may assume that you are using a large excess of benzene as your starting material.
C
:0
T
Add/Remove step
G
Chapter 13 Solutions
Chemistry: Structure and Properties
Ch. 13 - Determine what state this substance is in at 1 atm...Ch. 13 - Prob. 2SAQCh. 13 - Prob. 3SAQCh. 13 - Prob. 4SAQCh. 13 - Prob. 5SAQCh. 13 - Prob. 6SAQCh. 13 - Prob. 7SAQCh. 13 - Prob. 8SAQCh. 13 - Prob. 9SAQCh. 13 - Prob. 10SAQ
Ch. 13 - What is a phase diagram?Ch. 13 - Draw a generic phase diagram and label its...Ch. 13 - What is the significance of crossing a line in a...Ch. 13 - What is graphene? Why is graphene unique?Ch. 13 - Prob. 5ECh. 13 - What is a crystalline lattice? How is the lattice...Ch. 13 - Prob. 7ECh. 13 - Prob. 8ECh. 13 - What is the difference between hexagonal closest...Ch. 13 - What are the three basic types of solids and the...Ch. 13 - Prob. 11ECh. 13 - What kinds of forces hold each of the three basic...Ch. 13 - Prob. 13ECh. 13 - Prob. 14ECh. 13 - Prob. 15ECh. 13 - Prob. 16ECh. 13 - Prob. 17ECh. 13 - Prob. 18ECh. 13 - Prob. 19ECh. 13 - Consider the phase diagram for iodine shown here....Ch. 13 - Prob. 21ECh. 13 - Prob. 22ECh. 13 - Prob. 23ECh. 13 - Prob. 24ECh. 13 - Prob. 25ECh. 13 - An X-ray beam of unknown wavelength is diffracted...Ch. 13 - Prob. 27ECh. 13 - Determine the coordination number for each...Ch. 13 - Prob. 29ECh. 13 - Molybdenum crystallizes with the body-centred unit...Ch. 13 - Prob. 31ECh. 13 - An atom has a radius of 142 pm and crystallizes in...Ch. 13 - Rhodium has a density of 12.41 g / cm3 and...Ch. 13 - Barium has a density of 3.59 g/cm3 and...Ch. 13 - Prob. 35ECh. 13 - Palladium crystallizes with a face-centered cubic...Ch. 13 - Prob. 37ECh. 13 - Identify each solid as molecular, ionic, or...Ch. 13 - Which solid has the highest melting point? Why?...Ch. 13 - Which solid has the highest melting point? Why?...Ch. 13 - Which solid in each pair has the higher melting...Ch. 13 - Which solid in each pair has the higher melting...Ch. 13 - Prob. 43ECh. 13 - Prob. 44ECh. 13 - Prob. 45ECh. 13 - Prob. 46ECh. 13 - The unit cells for cesium chloride and barium(ll)...Ch. 13 - Prob. 48ECh. 13 - Prob. 49ECh. 13 - Prob. 50ECh. 13 - Prob. 51ECh. 13 - Prob. 52ECh. 13 - Prob. 53ECh. 13 - Prob. 54ECh. 13 - Prob. 55ECh. 13 - Prob. 56ECh. 13 - Prob. 57ECh. 13 - The density of an unknown metal is 12.3 g/cm3 and...Ch. 13 - Prob. 59ECh. 13 - Consider a planet where the pressure of the...Ch. 13 - An unknown metal is found to have a density of...Ch. 13 - Prob. 62ECh. 13 - Potassium chloride crystallizes in the rock salt...Ch. 13 - Calculate the fraction of empty space in cubic...Ch. 13 - Prob. 65ECh. 13 - Prob. 66ECh. 13 - Prob. 67E
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The following equations represent the formation of compound MX. What is the AH for the electron affinity of X (g)? X₂ (g) → 2X (g) M (s) → M (g) M (g) M (g) + e- AH = 60 kJ/mol AH = 22 kJ/mol X (g) + e-X (g) M* (g) +X (g) → MX (s) AH = 118 kJ/mol AH = ? AH = -190 kJ/mol AH = -100 kJ/mol a) -80 kJ b) -30 kJ c) -20 kJ d) 20 kJ e) 156 kJarrow_forwardA covalent bond is the result of the a) b) c) d) e) overlap of two half-filled s orbitals overlap of a half-filled s orbital and a half-filled p orbital overlap of two half-filled p orbitals along their axes parallel overlap of two half-filled parallel p orbitals all of the abovearrow_forwardCan the target compound at right be efficiently synthesized in good yield from the unsubstituted benzene at left? starting material target If so, draw a synthesis below. If no synthesis using reagents ALEKS recognizes is possible, check the box under the drawing area. Be sure you follow the standard ALEKS rules for submitting syntheses. + More... Note for advanced students: you may assume that you are using a large excess of benzene as your starting material. C T Add/Remove step X ноarrow_forward
- Which one of the following atoms should have the largest electron affinity? a) b) c) d) 으으 e) 1s² 2s² 2p6 3s¹ 1s² 2s² 2p5 1s² 2s² 2p 3s² 3p² 1s² 2s 2p 3s² 3p6 4s2 3ds 1s² 2s² 2p6arrow_forwardAll of the following are allowed energy levels except _. a) 3f b) 1s c) 3d d) 5p e) 6sarrow_forwardA student wants to make the following product in good yield from a single transformation step, starting from benzene. Add any organic reagents the student is missing on the left-hand side of the arrow, and any addition reagents that are necessary above or below the arrow. If this product can't be made in good yield with a single transformation step, check the box below the drawing area. Note for advanced students: you may assume that an excess of benzene is used as part of the reaction conditions. : ☐ + I X This product can't be made in a single transformation step.arrow_forward
- Ppplllleeeaaasssseeee helllppp wiithhh thisss Organic chemistryyyyyy I talked like this because AI is very annoyingarrow_forwardName the family to which each organic compound belongs. The first answer has been filled in for you. compound CH₂ || CH3-C-NH2 0 ။ CH3-C-CH₂ CH=O–CH=CH, CH₂ HO CH2-CH2-CH-CH3 family amine Darrow_forward1b. Br LOHarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax
Viscosity, Cohesive and Adhesive Forces, Surface Tension, and Capillary Action; Author: Professor Dave Explains;https://www.youtube.com/watch?v=P_jQ1B9UwpU;License: Standard YouTube License, CC-BY