
Concept explainers
(a)
Interpretation:
Whether hydration of propene will give one or two products has to be identified based on Markovnikov’s rule.
Concept Introduction:
In this reaction no atoms or group of atoms are removed. Instead the unsaturated bond is reduced to saturated bond. A general scheme for addition reaction of
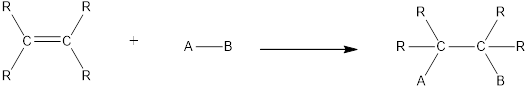
Addition reactions can be classified broadly into two types. They are asymmetrical addition reaction and symmetrical addition reaction.
Symmetrical addition reactions is the one in which the same atom or same group of atoms are added across the carbon‑carbon multiple bonds.
Unsymmetrical addition reactions is the one in which the different atom or different group of atoms are added across the carbon‑carbon multiple bonds.
Markovnikov’s rule:
When an unsymmetrical molecule of formula HQ to an unsymmeterical alkene, the hydrogen atom from HQ gets attached to the unsaturated carbon atom which has the most hydrogen atoms. In other words, it can be said that the hydrogen atom gets attached to the unsaturated carbon atom that is least substituted.
(b)
Interpretation:
Whether hydration of 3-hexene will give one or two products has to be identified based on Markovnikov’s rule.
Concept Introduction:
Chemical reaction in which an atom or a group of atoms are added to each carbon atom of a carbon‑carbon multiple bond in a hydrocarbon or hydrocarbon derivative is known as addition reaction.
In this reaction no atoms or group of atoms are removed. Instead the unsaturated bond is reduced to saturated bond. A general scheme for addition reaction of alkene can be given as shown below,

Addition reactions can be classified broadly into two types. They are asymmetrical addition reaction and symmetrical addition reaction.
Symmetrical addition reactions is the one in which the same atom or same group of atoms are added across the carbon‑carbon multiple bonds.
Unsymmetrical addition reactions is the one in which the different atom or different group of atoms are added across the carbon‑carbon multiple bonds.
Markovnikov’s rule:
When an unsymmetrical molecule of formula HQ to an unsymmeterical alkene, the hydrogen atom from HQ gets attached to the unsaturated carbon atom which has the most hydrogen atoms. In other words, it can be said that the hydrogen atom gets attached to the unsaturated carbon atom that is least substituted.
(c)
Interpretation:
Whether hydration of cyclopropene will give one or two products has to be identified based on Markovnikov’s rule.
Concept Introduction:
Chemical reaction in which an atom or a group of atoms are added to each carbon atom of a carbon‑carbon multiple bond in a hydrocarbon or hydrocarbon derivative is known as addition reaction.
In this reaction no atoms or group of atoms are removed. Instead the unsaturated bond is reduced to saturated bond. A general scheme for addition reaction of alkene can be given as shown below,

Addition reactions can be classified broadly into two types. They are asymmetrical addition reaction and symmetrical addition reaction.
Symmetrical addition reactions is the one in which the same atom or same group of atoms are added across the carbon‑carbon multiple bonds.
Unsymmetrical addition reactions is the one in which the different atom or different group of atoms are added across the carbon‑carbon multiple bonds.
Markovnikov’s rule:
When an unsymmetrical molecule of formula HQ to an unsymmeterical alkene, the hydrogen atom from HQ gets attached to the unsaturated carbon atom which has the most hydrogen atoms. In other words, it can be said that the hydrogen atom gets attached to the unsaturated carbon atom that is least substituted.
(d)
Interpretation:
Whether hydration of cyclopentene will give one or two products has to be identified based on Markovnikov’s rule.
Concept Introduction:
Chemical reaction in which an atom or a group of atoms are added to each carbon atom of a carbon‑carbon multiple bond in a hydrocarbon or hydrocarbon derivative is known as addition reaction.
In this reaction no atoms or group of atoms are removed. Instead the unsaturated bond is reduced to saturated bond. A general scheme for addition reaction of alkene can be given as shown below,

Addition reactions can be classified broadly into two types. They are asymmetrical addition reaction and symmetrical addition reaction.
Symmetrical addition reactions is the one in which the same atom or same group of atoms are added across the carbon‑carbon multiple bonds.
Unsymmetrical addition reactions is the one in which the different atom or different group of atoms are added across the carbon‑carbon multiple bonds.
Markovnikov’s rule:
When an unsymmetrical molecule of formula HQ to an unsymmeterical alkene, the hydrogen atom from HQ gets attached to the unsaturated carbon atom which has the most hydrogen atoms. In other words, it can be said that the hydrogen atom gets attached to the unsaturated carbon atom that is least substituted.
Want to see the full answer?
Check out a sample textbook solution
Chapter 13 Solutions
Study Guide with Selected Solutions for Stoker's General, Organic, and Biological Chemistry, 7th
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,





