![OWLv2 for Ebbing/Gammon's General Chemistry, 11th Edition, [Instant Access], 1 term (6 months)](https://s3.amazonaws.com/compass-isbn-assets/textbook_empty_images/large_textbook_empty.svg)
OWLv2 for Ebbing/Gammon's General Chemistry, 11th Edition, [Instant Access], 1 term (6 months)
11th Edition
ISBN: 9781305673939
Author: Darrell Ebbing; Steven D. Gammon
Publisher: Cengage Learning US
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 13, Problem 13.36QP
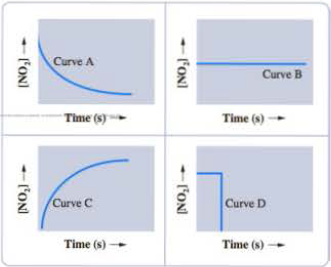
You carry out the following reaction by introducing N2O4 into an evacuated flask and observing the concentration change of the product over time.
Which one of the curves shown here reflects the data collected for this reaction?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
What is the molarity of a 0.393 m glucose solution if its density is 1.16 g/mL? MM glucose 180.2 g/mol
The rate constant for the decay of a radioactive element is 2.28 × 10⁻³ day⁻¹. What is the half-life of this element in days?
Handwritten please
Chapter 13 Solutions
OWLv2 for Ebbing/Gammon's General Chemistry, 11th Edition, [Instant Access], 1 term (6 months)
Ch. 13.1 - For the reaction given in Example 13.1, how is the...Ch. 13.1 - Iodide ion is oxidized by hypochlorite ion in...Ch. 13.1 - Shown here is a plot of the concentration of a...Ch. 13.3 - Prob. 13.3ECh. 13.3 - Prob. 13.2CCCh. 13.3 - Prob. 13.4ECh. 13.3 - Prob. 13.3CCCh. 13.4 - a. What would be the concentration of dinitrogen...Ch. 13.4 - The isomerization of cyclopropane, C3H6, to...Ch. 13.4 - A reaction believed to be either first or second...
Ch. 13.5 - Consider the following potential-energy curves for...Ch. 13.6 - Acetaldehyde, CH3CHO, decomposes when heated....Ch. 13.7 - Prob. 13.8ECh. 13.7 - Prob. 13.9ECh. 13.7 - Prob. 13.10ECh. 13.8 - The iodide-ion-catalyzed decomposition of hydrogen...Ch. 13.8 - Prob. 13.12ECh. 13.8 - Prob. 13.6CCCh. 13 - List the four variables or factors that can affect...Ch. 13 - Define the rate of reaction of HBr in the...Ch. 13 - Give at least two physical properties that might...Ch. 13 - A rate of reaction depends on four variables...Ch. 13 - Prob. 13.5QPCh. 13 - The reaction...Ch. 13 - The rate of a reaction is quadrupled when the...Ch. 13 - Prob. 13.8QPCh. 13 - The reaction A(g)B(g)+C(g) is known to be first...Ch. 13 - Prob. 13.10QPCh. 13 - Prob. 13.11QPCh. 13 - Sketch a potential-energy diagram for the...Ch. 13 - Draw a structural formula for the activated...Ch. 13 - Prob. 13.14QPCh. 13 - Prob. 13.15QPCh. 13 - Prob. 13.16QPCh. 13 - Prob. 13.17QPCh. 13 - Prob. 13.18QPCh. 13 - The dissociation of N2O4 into NO2, N2O4(g)2NO2(g)...Ch. 13 - Prob. 13.20QPCh. 13 - Prob. 13.21QPCh. 13 - Prob. 13.22QPCh. 13 - You are running the reaction 2A+BC+3D. Your lab...Ch. 13 - At a constant temperature, which of the following...Ch. 13 - Consider the reaction E+FG+H, which has the...Ch. 13 - The hypothetical reaction A+B+CD+E has the rate...Ch. 13 - Kinetics I Consider the hypothetical reaction A(g)...Ch. 13 - Kinetics II You and a friend are working together...Ch. 13 - Consider the reaction 3A2B+C. a One rate...Ch. 13 - Given the reaction 2A+BC+3D, can you write the...Ch. 13 - The reaction 2A(g)A2(g) is being run in each of...Ch. 13 - Prob. 13.32QPCh. 13 - You perform some experiments for the reaction AB+C...Ch. 13 - A friend of yours runs a reaction and generates...Ch. 13 - Prob. 13.35QPCh. 13 - You carry out the following reaction by...Ch. 13 - Prob. 13.37QPCh. 13 - The chemical reaction AB+C has a rate constant...Ch. 13 - Relate the rate of decomposition of NH4NO2 to the...Ch. 13 - For the reaction of hydrogen with iodine...Ch. 13 - To obtain the rate of the reaction...Ch. 13 - To obtain the rate of the reaction...Ch. 13 - Ammonium nitrite, NH4NO2, decomposes in solution,...Ch. 13 - Iron(III) chloride is reduced by tin(II) chloride....Ch. 13 - Azomethane, CH3NNCH3, decomposes according to the...Ch. 13 - Nitrogen dioxide, NO2, decomposes upon heating to...Ch. 13 - Hydrogen sulfide is oxidized by chlorine in...Ch. 13 - For the reaction of nitrogen monoxide, NO, with...Ch. 13 - Prob. 13.49QPCh. 13 - Prob. 13.50QPCh. 13 - In experiments on the decomposition of azomethane....Ch. 13 - Ethylene oxide. C2H4O, decomposes when heated to...Ch. 13 - Nitrogen monoxide NO, reacts with hydrogen to give...Ch. 13 - In a kinetic study of the reaction...Ch. 13 - Chlorine dioxide, ClO2, is a reddish-yellow gas...Ch. 13 - Iodide ion is oxidized to hypoiodite ion, IO, by...Ch. 13 - Sulfuryl chloride, SO2Cl2, decomposes when heated....Ch. 13 - Cyclopropane, C3H6, is converted to its isomer...Ch. 13 - A reaction of the form aA Products is second-order...Ch. 13 - A reaction of the form aA Products is second order...Ch. 13 - Ethyl chloride, CH3CH2Cl, used to produce...Ch. 13 - Cyclobutane, C4H8, consisting of molecules in...Ch. 13 - Methyl isocyanide, CH3NC, isomerizes, when heated,...Ch. 13 - Dinitrogen pentoxide, N2O5, decomposes when heated...Ch. 13 - In the presence of excess thiocyanate ion, SCN,...Ch. 13 - In the presence of excess thiocyanate ion, SCN,...Ch. 13 - A reaction of the form aA Products is second order...Ch. 13 - A reaction of the form aA Products is second order...Ch. 13 - In the presence of excess thiocyanate ion, SCN,...Ch. 13 - In the presence of excess thiocyanate ion, SCN,...Ch. 13 - It is found that a gas undergoes a zero-order...Ch. 13 - The reaction AB+C is found to be zero order. If it...Ch. 13 - Chlorine dioxide oxidizes iodide ion in aqueous...Ch. 13 - Methyl acetate, CH3COOCH3, reacts in basic...Ch. 13 - Sketch a potential-energy diagram for the reaction...Ch. 13 - Sketch a potential-energy diagram for the...Ch. 13 - In a series of experiments on the decomposition of...Ch. 13 - The reaction 2NOCl(g)2NO(g)+Cl2(g) has...Ch. 13 - The rate of a particular reaction increases by a...Ch. 13 - The rate of a particular reaction quadruples when...Ch. 13 - The following values of the rate constant were...Ch. 13 - The following values of the rate constant were...Ch. 13 - Nitrogen monoxide, NO, is believed to react with...Ch. 13 - The decomposition of ozone is believed to occur in...Ch. 13 - Identify the molecularity of each of the following...Ch. 13 - Prob. 13.86QPCh. 13 - Write a rate equation, showing the dependence of...Ch. 13 - Prob. 13.88QPCh. 13 - The isomerization of cyclopropane, C3H6, is...Ch. 13 - The thermal decomposition of nitryl chloride,...Ch. 13 - The reaction H2(g)+I2(g)2HI(g) may occur by the...Ch. 13 - Ozone decomposes to oxygen gas. 2O3(g)3O2(g) A...Ch. 13 - The following is a possible mechanism for a...Ch. 13 - Consider the following mechanism for a reaction in...Ch. 13 - A study of the decomposition of azomethane,...Ch. 13 - Nitrogen dioxide decomposes when heated....Ch. 13 - Prob. 13.97QPCh. 13 - Prob. 13.98QPCh. 13 - Methyl acetate reacts in acidic solution....Ch. 13 - Benzene diazonium chloride, C6H5NNCl, decomposes...Ch. 13 - What is the half-life of methyl acetate hydrolysis...Ch. 13 - What is the half-life of benzene diazonium...Ch. 13 - A compound decomposes by a first-order reaction....Ch. 13 - A compound decomposes by a first-order reaction....Ch. 13 - Butadiene can undergo the following reaction to...Ch. 13 - Prob. 13.106QPCh. 13 - Prob. 13.107QPCh. 13 - A second-order decomposition reaction run at 550oC...Ch. 13 - Prob. 13.109QPCh. 13 - Prob. 13.110QPCh. 13 - Prob. 13.111QPCh. 13 - Prob. 13.112QPCh. 13 - The decomposition of nitrogen dioxide,...Ch. 13 - Prob. 13.114QPCh. 13 - Prob. 13.115QPCh. 13 - Prob. 13.116QPCh. 13 - Nitryl bromide, NO2Br, decomposes into nitrogen...Ch. 13 - Tertiary butyl chloride reacts in basic solution...Ch. 13 - Urea, (NH2)2CO, can be prepared by heating...Ch. 13 - Prob. 13.120QPCh. 13 - A study of the gas-phase oxidation of nitrogen...Ch. 13 - The reaction of water with CH3Cl in acetone as a...Ch. 13 - The reaction of thioacelamidc with water is shown...Ch. 13 - Prob. 13.124QPCh. 13 - Prob. 13.125QPCh. 13 - Prob. 13.126QPCh. 13 - Prob. 13.127QPCh. 13 - Prob. 13.128QPCh. 13 - Prob. 13.129QPCh. 13 - Prob. 13.130QPCh. 13 - The rate constant for a certain reaction is 1.4 ...Ch. 13 - The decomposition of hydrogen peroxide is a first...Ch. 13 - Prob. 13.133QPCh. 13 - What is the rate law for the following gas-phase...Ch. 13 - A possible mechanism for a gas-phase reaction is...Ch. 13 - Say you run the following elementary, termolecular...Ch. 13 - Prob. 13.137QPCh. 13 - For the decomposition of one mole of nitrosyl...Ch. 13 - Given the following mechanism for a chemical...Ch. 13 - The following data were collected for the reaction...Ch. 13 - A hypothetical reaction has the two-step mechanism...Ch. 13 - Prob. 13.142QPCh. 13 - Prob. 13.143QPCh. 13 - Prob. 13.144QPCh. 13 - Dinitrogen pentoxide decomposes according to the...Ch. 13 - Prob. 13.146QPCh. 13 - Dinitrogen pentoxide, N2O5, undergoes first-order...Ch. 13 - Prob. 13.148QPCh. 13 - Hydrogen peroxide in aqueous solution decomposes...Ch. 13 - Nitrogen dioxide reacts with carbon monoxide by...Ch. 13 - Nitrogen monoxide reacts with oxygen to give...Ch. 13 - Nitrogen monoxide reacts with hydrogen as follows:...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Choose the best reagents to complete the following reaction. i H A B 1. CH3CH2Na 2. H3O+ 1. CH3CH2MgBr 2. H3O+ 1. CH3MgBr Q C 2. H3O+ 1. H3O+ D 2. CH3MgBr 00 OH Q E CH³MgBrarrow_forwardThe kinetics of a gas phase reaction of the form A → Products results in a rate constant of 0.00781 M/min. For this reaction, the initial concentration of A is 0.501 M. What is the half-life for this reaction?arrow_forwardChoose the best reagents to complete the following reaction. 1. PhNa A 2. H3O+ 1. PhCH2MgBr B 2. H3O+ хё 1. PhMgBr C 2. H3O+ 00 HO Q E D 1. H3O+ 2. PhMgBr PhMgBrarrow_forward
- Please answer all of the questions and provide detailed explanations and include a drawing to show the different signals on the molecule and include which ones should be highlighted.arrow_forwardDraw the major product of this reaction. Ignore inorganic byproducts. Incorrect, 1 attempt remaining 1. LiAlH4 2. H3O+ Q OH ☑ Select to Drawarrow_forwardHow should I graph my data for the Absorbance of Pb and Fe for each mushroom? I want to compare the results to the known standard curve. Software: Excel Spreadsheets Link: https://mnscu-my.sharepoint.com/:x:/g/personal/vi2163ss_go_minnstate_edu/Eb2PfHdfEtBJiWh0ipHZ_kkBW4idWWwvpLPPtqoq2WkgbQ?rtime=HxrF0_tR3Ugarrow_forward
- Provide the proper IUPAC name only for the following compound. Dashes, commas, and spaces must be used correctly, but do not use italics in Canvas.arrow_forwardThe kinetics of a gas phase reaction of the form A → Products results in a rate constant of 0.00781 M/min. For this reaction, the initial concentration of A is 0.501 M. How many minutes will it take for the concentration of A to reach 0.144 Marrow_forwardWhat is the rate for the second order reaction A → Products when [A] = 0.256 M? (k = 0.761 M⁻¹s⁻¹)arrow_forward
- For reaction N2(g) + O2(g) --> 2NO(g) Write the rate of the reaction in terms of change of NO.arrow_forwardDon't used hand raiting and don't used Ai solutionarrow_forwardThe reaction of 2-oxacyclopentanone with hydrochloric acid in water (i.e., "excess") produces which of the following carboxylic acids?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning, Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning


Physical Chemistry
Chemistry
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Wadsworth Cengage Learning,

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning
Kinetics: Chemistry's Demolition Derby - Crash Course Chemistry #32; Author: Crash Course;https://www.youtube.com/watch?v=7qOFtL3VEBc;License: Standard YouTube License, CC-BY