
EP ESSENTIAL ORG.CHEM.-MOD.MASTERING
3rd Edition
ISBN: 9780133858501
Author: Bruice
Publisher: PEARSON CO
expand_more
expand_more
format_list_bulleted
Question
Chapter 12.8, Problem 22P
(a)
Interpretation Introduction
Interpretation:
The carbonyl compound should be identified for the given amino acid preparation.
Concept introduction:
Amino acid:
An amino acid is an organic molecule and it consists of
The general formula for the amino acid is given below,
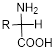
(b)
Interpretation Introduction
Interpretation:
The carbonyl compound should be identified for the given amino acid preparation.
Concept introduction:
Amino acid:
An amino acid is an organic molecule and it consists of amine group
The general formula for the amino acid is given below,
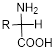
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
I
Draw the anti-Markovnikov product of the hydration of this alkene.
this problem.
Note for advanced students: draw only one product, and don't worry about showing any stereochemistry. Drawing dash and wedge bonds has been disabled for
esc
esc
☐
Explanation
Check
F1
1
2
F2
# 3
F3
+
$
14
×
1. BH THE
BH3
2. H O NaOH
'2 2'
Click and drag to start
drawing a structure.
F4
Q
W
E
R
A
S
D
%
905
LL
F5
F6
F7
© 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility
<
&
6
7
27
8
T
Y
U
G
H
I
F8
F9
F10
F11
F12
9
0
J
K
L
P
+ //
command option
Z
X
C
V B
N
M
H
H
rol
option
command
AG/F-2° V
3. Before proceeding with this problem you may want to glance at p. 466 of your textbook
where various oxo-phosphorus derivatives and their oxidation states are summarized.
Shown below are Latimer diagrams for phosphorus at pH values at 0 and 14:
-0.93
+0.38
-0.50
-0.51 -0.06
H3PO4 →H4P206 →H3PO3 →→H3PO₂ → P → PH3
Acidic solution
Basic solution
-0.28
-0.50
3--1.12
-1.57
-2.05 -0.89
PO HPO H₂PO₂ →P → PH3
-1.73
a) Under acidic conditions, H3PO4 can be reduced into H3PO3 directly (-0.28V), or via the
formation and reduction of H4P206 (-0.93/+0.38V). Calculate the values of AG's for both
processes; comment.
(3 points)
0.5
PH
P
0.0
-0.5
-1.0-
-1.5-
-2.0
H.PO,
-2.3+
-3 -2
-1
1
2
3
2
H,PO,
b) Frost diagram for phosphorus under acidic
conditions is shown. Identify possible
disproportionation and comproportionation processes;
write out chemical equations describing them. (2 points)
H,PO
4
S
Oxidation stale, N
Chapter 12 Solutions
EP ESSENTIAL ORG.CHEM.-MOD.MASTERING
Ch. 12.1 - Prob. 1PCh. 12.1 - Name the following compounds:Ch. 12.1 - Prob. 3PCh. 12.1 - Prob. 4PCh. 12.2 - Prob. 5PCh. 12.4 - Draw the structure for each of the following: a....Ch. 12.4 - Prob. 7PCh. 12.5 - What products are formed when the following...Ch. 12.5 - Prob. 9PCh. 12.5 - Prob. 10P
Ch. 12.5 - Prob. 12PCh. 12.5 - Write the mechanism for the reaction of acetyl...Ch. 12.5 - Prob. 14PCh. 12.6 - Prob. 16PCh. 12.7 - Prob. 17PCh. 12.7 - Prob. 18PCh. 12.7 - Prob. 19PCh. 12.8 - Prob. 20PCh. 12.8 - Prob. 21PCh. 12.8 - Prob. 22PCh. 12.9 - Which of the following are a. hemiacetals? b....Ch. 12.9 - Prob. 24PCh. 12.9 - Prob. 25PCh. 12.10 - Prob. 26PCh. 12.11 - Prob. 27PCh. 12 - Draw the structure for each of the following: a....Ch. 12 - Prob. 29PCh. 12 - List the following compounds in order from most...Ch. 12 - Show the reagents required to form the primary...Ch. 12 - Fill in the boxes:Ch. 12 - Indicate how the following compounds could be...Ch. 12 - Prob. 34PCh. 12 - Prob. 35PCh. 12 - Prob. 36PCh. 12 - Prob. 37PCh. 12 - Prob. 38PCh. 12 - Prob. 39PCh. 12 - Show two ways the following compound could be...Ch. 12 - Prob. 41PCh. 12 - Prob. 42PCh. 12 - Prob. 43PCh. 12 - Prob. 44PCh. 12 - Prob. 45PCh. 12 - Prob. 46PCh. 12 - Prob. 47PCh. 12 - Prob. 48PCh. 12 - Prob. 49PCh. 12 - Prob. 50PCh. 12 - Prob. 51PCh. 12 - Indicate how the following compounds could be...Ch. 12 - Prob. 53P
Knowledge Booster
Similar questions
- 4. For the following complexes, draw the structures and give a d-electron count of the metal: a) Tris(acetylacetonato)iron(III) b) Hexabromoplatinate(2-) c) Potassium diamminetetrabromocobaltate(III) (6 points)arrow_forward2. Calculate the overall formation constant for [Fe(CN)6]³, given that the overall formation constant for [Fe(CN)6] 4 is ~1032, and that: Fe3+ (aq) + e = Fe²+ (aq) E° = +0.77 V [Fe(CN)6]³ (aq) + e¯ = [Fe(CN)6] (aq) E° = +0.36 V (4 points)arrow_forward5. Consider the compounds shown below as ligands in coordination chemistry and identify their denticity; comment on their ability to form chelate complexes. (6 points) N N A B N N N IN N Carrow_forward
- 1. Use standard reduction potentials to rationalize quantitatively why: (6 points) (a) Al liberates H2 from dilute HCl, but Ag does not; (b) Cl2 liberates Br2 from aqueous KBr solution, but does not liberate C12 from aqueous KCl solution; c) a method of growing Ag crystals is to immerse a zinc foil in an aqueous solution of AgNO3.arrow_forwardWhat would be the best choices for the missing reagents 1 and 3 in this synthesis? 1 1. PPh3 2. n-BuLi 3 2 • Draw the missing reagents in the drawing area below. You can draw them in any arrangement you like. • Do not draw the missing reagent 2. If you draw 1 correctly, we'll know what it is. • Note: if one of your reagents needs to contain a halogen, use bromine. Click and drag to start drawing a structure. Xarrow_forwardWhat is the missing reactant R in this organic reaction? N N H3O+ +R + • Draw the structure of R in the drawing area below. • Be sure to use wedge and dash bonds if it's necessary to draw one particular enantiomer. Click and drag to start drawing a structure. fmarrow_forward
- The product on the right-hand side of this reaction can be prepared from two organic reactants, under the conditions shown above and below the arrow. Draw 1 and 2 below, in any arrangement you like. 1+2 NaBH3CN H+ N Click and drag to start drawing a structure. 5arrow_forwardAssign this HSQC Spectrum ( please editing clearly on the image)arrow_forward(a 4 shows scanning electron microscope (SEM) images of extruded actions of packing bed for two capillary columns of different diameters, al 750 (bottom image) and b) 30-μm-i.d. Both columns are packed with the same stationary phase, spherical particles with 1-um diameter. A) When the columns were prepared, the figure shows that the column with the larger diameter has more packing irregularities. Explain this observation. B) Predict what affect this should have on band broadening and discuss your prediction using the van Deemter terms. C) Does this figure support your explanations in application question 33? Explain why or why not and make any changes in your answers in light of this figure. Figure 4 SEM images of sections of packed columns for a) 750 and b) 30-um-i.d. capillary columns.³arrow_forward
- fcrip = ↓ bandwidth Il temp 32. What impact (increase, decrease, or no change) does each of the following conditions have on the individual components of the van Deemter equation and consequently, band broadening? Increase temperature Longer column Using a gas mobile phase instead of liquid Smaller particle stationary phase Multiple Paths Diffusion Mass Transferarrow_forward34. Figure 3 shows Van Deemter plots for a solute molecule using different column inner diameters (i.d.). A) Predict whether decreasing the column inner diameters increase or decrease bandwidth. B) Predict which van Deemter equation coefficient (A, B, or C) has the greatest effect on increasing or decreasing bandwidth as a function of i.d. and justify your answer. Figure 3 Van Deemter plots for hydroquinone using different column inner diameters (i.d. in μm). The data was obtained from liquid chromatography experiments using fused-silica capillary columns packed with 1.0-μm particles. 35 20 H(um) 큰 20 15 90 0+ 1500 100 75 550 01 02 594 05 μ(cm/sec) 30 15 10arrow_forwardelow are experimentally determined van Deemter plots of column efficiency, H, vs. flow rate. H is a quantitative measurement of band broadening. The left plot is for a liquid chromatography application and the night is for gas chromatography. Compare and contrast these two plots in terms of the three band broadening mechanisms presented in this activity. How are they similar? How do they differ? Justify your answers.? 0.4 H (mm) 0.2 0.1- 0.3- 0 0.5 H (mm) 8.0 7.0 6.0 5.0 4.0- 3.0 T +++ 1.0 1.5 0 2.0 4.0 Flow Rate, u (cm/s) 6.0 8.0 Flow Rate, u (cm/s)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning