
(a)
Interpretation:
It should be determined that the amide used to produce Benzylmethylamine on reaction with
Concept introduction:
LiAH4 (Lithium aluminium hydride):
Lithium aluminium hydride is used as a reducing agent.
Lithium aluminium hydride is reduced the amide as an
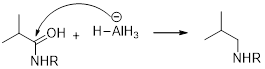
(b)
Interpretation:
It should be determined that the amide used to produce Ethylamine on rection with
Concept introduction:
LiAH4 (Lithium aluminium hydride):
Lithium aluminium hydride is used as a reducing agent.
Lithium aluminium hydride is reduced the amide as an amine which is shown below.
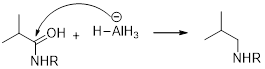
(c)
Interpretation:
It should be determined that the amide used to produce Diethylamine on rection with
Concept introduction:
LiAH4 (Lithium aluminium hydride):
Lithium aluminium hydride is used as a reducing agent.
Lithium aluminium hydride is reduced the amide as an amine which is shown below.
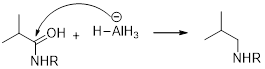
(d)
Interpretation:
It should be determined that the amide used to produce Triethylamine on rection with
Concept introduction:
LiAH4 (Lithium aluminium hydride):
Lithium aluminium hydride is used as a reducing agent.
Lithium aluminium hydride is reduced the amide as an amine which is shown below.
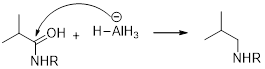
Want to see the full answer?
Check out a sample textbook solution
Chapter 12 Solutions
EP ESSENTIAL ORG.CHEM.-MOD.MASTERING
- Draw the Zaitsev product of the dehydration of this alcohol. + I X 5 OH ざ~ TSOH Click and drag to start drawing a structure.arrow_forwardPlease help with identifying these.arrow_forwardFor the reaction: CO2(g) + H2(g) --> CO (g) + H2O (g) Kc= 0.64 at 900 degrees celcius. if initially you start with 1.00 atmoshpere of carbon dioxide and 1 atmoshpere of hydrogen gas, what are the equilibrium partial pressuses of all species.arrow_forward
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning





