
(a)
Interpretation: The major product of the given reaction has to be found.
Concept Introduction:
Major product in the reaction of
General scheme:
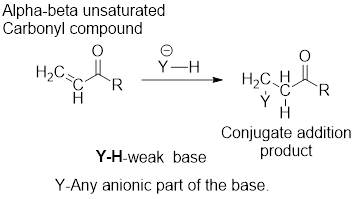
It can be observed that the conjugate addition product has been formed by the addition of basic anion to the beta-carbon atom and that of hydrogen atom to the alpha-carbon carbon atom.
The examples for the weak bases are:
(b)
Interpretation: The major product of the given reaction has to be found.
Concept Introduction:
Major product in the reaction of
General scheme:

It can be observed that the conjugate addition product has been formed by the addition of basic anion to the beta-carbon atom and that of hydrogen atom to the alpha-carbon carbon atom.
The examples for the weak bases are:
(c)
Interpretation: The major product of the given reaction has to be found.
Concept Introduction:
Major product in the reaction of
General scheme:

It can be observed that the conjugate addition product has been formed by the addition of basic anion to the beta-carbon atom and that of hydrogen atom to the alpha-carbon carbon atom.
The examples for the weak bases are:
Major product in the reaction of
General scheme:
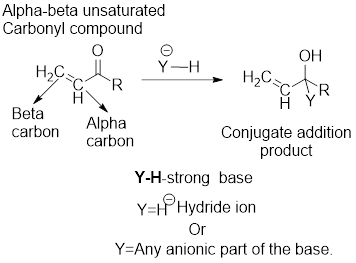
It can be observed that the direct addition product has been formed from the direct reaction of the strong base with the carbonyl
The examples for the strong bases are:
(d)
Interpretation: The major product of the given reaction has to be found.
Concept Introduction:
Major product in the reaction of
General scheme:

It can be observed that the conjugate addition product has been formed by the addition of basic anion to the beta-carbon atom and that of hydrogen atom to the alpha-carbon carbon atom.
The examples for the weak bases are:
Major product in the reaction of
General scheme:

It can be observed that the direct addition product has been formed from the direct reaction of the strong base with the carbonyl functional group of the
The examples for the strong bases are:
Want to see the full answer?
Check out a sample textbook solution
Chapter 12 Solutions
EP ESSENTIAL ORG.CHEM.-MOD.MASTERING
- How to draw the mechanism for this reaction?arrow_forward> H₂C=C-CH2-CH3 B. H₂O Pt C. + H2 + H₂O H D. 16. Give the IUPAC name for each of the following: B. Cl Cl c. Cl Cl 17. Draw the line-angle formula for each of the following compounds: 1. phenol 2. 1,3-dichlorobenzene 3. 4-ethyltoluene < Previous Submit Assignment Next ▸arrow_forwardno Ai walkthroughsarrow_forward
- The answer is shown. What is the reaction mechanism to arrive at the answer?arrow_forwardno Ai walkthroughsarrow_forwardConsider the following nucleophilic substitution reaction. The compound listed above the arrow is the solvent for the reaction. If nothing is listed over the arrow, then the nucleophile is also the solvent for the reaction. Part 1 of 2 Br CH,CN + I¯ What is the correct mechanism for the reaction? Select the single best answer. @SN2 ○ SN 1 Part: 1/2 Part 2 of 2 Draw the products for the reaction. Include both the major organic product and the inorganic product. If more than one stereoisomer is possible, draw only one stereoisomer. Include stereochemistry where relevant. Click and drag to start drawing a structure. X હૈarrow_forward
- 20.33 Think-Pair-Share (a) Rank the following dienes and dienophiles in order of increasing reactivity in the Diels-Alder reaction. (i) CO₂Et (ii) COEt || CO₂Et MeO MeO (b) Draw the product that results from the most reactive diene and most reactive dienophile shown in part (a). (c) Draw a depiction of the orbital overlap involved in the pericyclic reaction that oc- curs between the diene and dienophile in part (b). (d) Is the major product formed in part (b) the endo or exo configuration? Explain your reasoning.arrow_forward20.40 The following compound undergoes an intramolecular Diels-Alder reaction to give a tricyclic product. Propose a structural formula for the product. CN heat An intramolecular Diels-Alder adductarrow_forwardWhat is the reaction mechanism for this? Can this even be done without a base?arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
