
Concept explainers
Methane is to be adiabatically and reversibly compressed from 50 psia and 100°F to 500 psia. Calculate the specific work required for this compression treating the methane as an ideal gas with variable specific heats and using the departure charts.
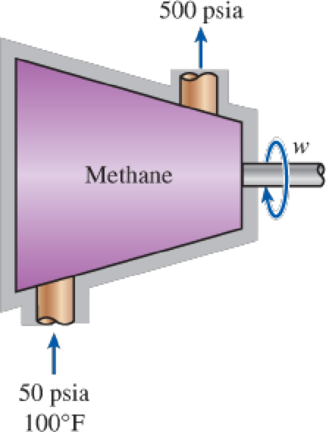
FIGURE P12–96E
The work input of the compressor per unit mass by treating the methane as an ideal gas with variable specific heats and using departure charts.
Answer to Problem 96RP
The work input of the compressor per unit mass by treating the methane as an ideal gas with variable specific heats is
The work input of the compressor per unit mass of the methane using departure charts is
Explanation of Solution
Refer the table A-2E (c), “Ideal gas specific heats of various common gases”.
The general empirical correlation is
Write the formula for enthalpy change in molar basis at ideal gas state
Here, specific heat capacity at constant pressure is
Write the formula for work input to the compressor
Here, molar mass of methane is
Write the formula for entropy change in molar basis at ideal gas state
Here, universal gas constant is
Calculate the reduced temperature
Here, critical temperature is
Calculate the reduced pressure
Here, critical pressure is
Calculate the reduced temperature
Here, critical temperature is
Calculate the reduced pressure
Here, critical pressure is
Write the formula for change in enthalpy
Here, change in enthalpy of ideal gas is
Refer Table A-1E, “Molar mass, gas constant, and critical-point properties”.
The critical temperature and critical pressure of the methane is as follows.
Refer table A-1E, “Molar mass, gas constant and critical properties table”.
The molar mass
Refer the table A-2E (c), “Ideal gas specific heats of various common gases”.
Obtain the empirical constants as follows.
Refer Table A-2E (a), “Ideal-gas specific heats of various common gases”.
The gas constant
The specific heat at constant pressure
The universal gas constant
Conclusion:
Convert the inlet temperature from degree Fahrenheit to Rankine.
It is given that the compression process in reversible adiabatic process. Hence, the change in entropy during the process is zero.
Substitute
By using Engineering Equation Solver (EES) or online calculator to solve the Equation (XI) and obtain the value of
Substitute
Substitute
Thus, the work input of the compressor per unit mass by treating the methane as an ideal gas with variable specific heats is
Substitute
Substitute
Refer Figure A-29, “Generalized enthalpy departure chart”.
The enthalpy departure factor
Consider the final temperature
Substitute
Substitute
Refer Figure A-29, “Generalized enthalpy departure chart”.
The enthalpy departure factor
Substitute
Here, the work input of the compressor is equal to the enthalpy difference.
Thus, the work input of the compressor per unit mass of the methane using departure charts is
Want to see more full solutions like this?
Chapter 12 Solutions
Thermodynamics: An Engineering Approach ( 9th International Edition ) ISBN:9781260092684
- 20. [Ans. 9; 71.8 mm] A semi-elliptical laminated spring is made of 50 mm wide and 3 mm thick plates. The length between the supports is 650 mm and the width of the band is 60 mm. The spring has two full length leaves and five graduated leaves. If the spring carries a central load of 1600 N, find: 1. Maximum stress in full length and graduated leaves for an initial condition of no stress in the leaves. 2. The maximum stress if the initial stress is provided to cause equal stress when loaded. [Ans. 590 MPa ; 390 MPa ; 450 MPa ; 54 mm] 3. The deflection in parts (1) and (2).arrow_forwardQ6/ A helical square section spring is set inside another, the outer spring having a free length of 35 mm greater than the inner spring. The dimensions of each spring are as follows: Mean diameter (mm) Side of square section (mm) Active turns Outer Inner Spring Spring 120 70 8 7 20 15 Determine the (1) Maximum deflection of the two springs and (2) Equivalent spring rate of the two springs after sufficient load has been applied to deflect the outer spring 60 mm. Use G = 83 GN/m².arrow_forwardQ2/ The bumper springs of a railway carriage are to be made of rectangular section wire. The ratio of the longer side of the wire to its shorter side is 1.5, and the ratio of mean diameter of spring to the longer side of wire is nearly equal to 6. Three such springs are required to bring to rest a carriage weighing 25 kN moving with a velocity of 75 m/min with a maximum deflection of 200 mm. Determine the sides of the rectangular section of the wire and the mean diameter of coils when the shorter side is parallel to the axis of the spring. The allowable shear stress is not to exceed 300 MPa and G = 84 kN/mm². Q6/ A belicalarrow_forward
- 11. A load of 2 kN is dropped axially on a close coiled helical spring, from a height of 250 mm. The spring has 20 effective turns, and it is made of 25 mm diameter wire. The spring index is 8. Find the maximum shear stress induced in the spring and the amount of compression produced. The modulus of rigidity for the material of the spring wire is 84 kN/mm². [Ans. 287 MPa; 290 mm]arrow_forwardWhat is the reason for this composition?arrow_forwardHomework: ANOVA Table for followed design B AB Dr -1 -1 1 (15.18,12) 1 -1 -1 (45.48.51) -1 1 -1 (25,28,19) 1 1 (75.75,81)arrow_forward
- S B Pin 6 mm Garrow_forwardMid-Term Exam 2024/2025 Post graduate/Applied Mechanics- Metallurgy Q1/ State the type of fault in the following case, and state the structure in which it will appear. АВСАВСВАСВАСАВСАВСarrow_forwardالثانية Babakt Momentum equation for Boundary Layer S SS -Txfriction dray Momentum equation for Boundary Layer What laws are important for resolving issues 2 How to draw. 3 What's Point about this.arrow_forward
- R αι g The system given on the left, consists of three pulleys and the depicted vertical ropes. Given: ri J₁, m1 R = 2r; απ r2, J2, m₂ m1; m2; M3 J1 J2 J3 J3, m3 a) Determine the radii 2 and 3.arrow_forwardB: Solid rotating shaft used in the boat with high speed shown in Figure. The amount of power transmitted at the greatest torque is 224 kW with 130 r.p.m. Used DE-Goodman theory to determine the shaft diameter. Take the shaft material is annealed AISI 1030, the endurance limit of 18.86 kpsi and a factor of safety 1. Which criterion is more conservative? Note: all dimensions in mm. 1 AA Motor 300 Thrust Bearing Sprocket 100 9750 เอarrow_forwardQ2: The plate material of a pressure vessel is AISI 1050 QT 205 °C. The plate is rolled to a diameter of 1.2 m. The two sides of the plate are connected via a riveted joint as shown below. If the rivet material is G10500 with HB=197 and all rivet sizes M31. Find the required rivet size when the pressure vessel is subjected to an internal pressure of 500 MPa. Take safety factor = 2. 1.2m A B' A Chope olm 10.5 0.23 hopearrow_forward
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





