
(a)
Interpretation:Name of element from below valence electron diagram should be identified.
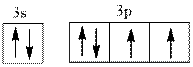
Concept introduction:The process by which electrons are distributed in molecular or atomic orbitals is termed as electronic configuration. Hund’s rule of multiplicity states that electrons are filled singly in an orbital initially and then pairing occurs with different spin. Thus atomic orbital is filled in accordance to this rule.
Valence electron defines the group of the element. Energy, size, shape, and orientation of atomic orbital are determined with help of some numbers. These numbers are called quantum numbers. Principal quantum number is represented by
(b)
Interpretation:Name of element from below valence electron diagram shouldbe identified.
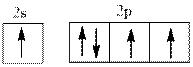
Concept introduction:The process by which electrons are distributed in molecular or atomic orbitals is termed as electronic configuration. Hund’s rule of multiplicity states that electrons are filled singly in an orbital initially and then pairing occurs with different spin. Thus atomic orbital is filled in accordance to this rule.
Valence electron defines the group of the element. Energy, size, shape, and orientation of atomic orbital are determined with help of some numbers. These numbers are called quantum numbers. Principal quantum number is represented by
(c)
Interpretation:Name of element from below valence electron diagram of ion shouldbe identified.
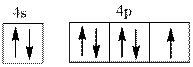
Concept introduction:The process by which electrons are distributed in molecular or atomic orbitals is termed as electronic configuration. Hund’s rule of multiplicity states that electrons are filled singly in an orbital initially and then pairing occurs with different spin. Thus atomic orbital is filled in accordance to this rule.
Valence electron defines the group of the element. Energy, size, shape, and orientation of atomic orbital are determined with help of some numbers. These numbers are called quantum numbers. Principal quantum number is represented by
Want to see the full answer?
Check out a sample textbook solution
Chapter 12 Solutions
Chemical Principles
- Indicate the product that is obtained if the benzotriazol reacts with dimethyl sulfate.arrow_forwardIndicate how to obtain 2-metilbencimidazol from 1,2-diaminobenzene.arrow_forwardbreak down both reactions shown and explain it correctly using the bromonium ion mechanism, instead of the (disproven) carbocation-based mechanism.arrow_forward
- Indicate how from 1,2-diaminobenzene to obtain 1-metilbenzotriazol.arrow_forward-C = C - C - + Br₂ + I" -> -C-C-c -C = C -C- + Br² + I₂ -C=C Br I + Brū + Iz -7- C - C-C- I Br Mechanism; - C = c - c - + Br - Br > - C-c-c- Br -C-C-C- + 1 - - -Ċ-Ċ'-c' - Br Br Iarrow_forwardWrite the mechanism of the esterification reaction (please show the mechanism included line pairs and arrows)arrow_forward
- How do I break down the reaction shown on the chalkboard and explain it correctly using the bromonium ion mechanism, instead of the (disproven) carbocation-based mechanismarrow_forward¿Qué the product is obtained from tetraethoxypropano and hidrazina?. Indicate the reason why the corresponding dial is used.arrow_forwardIf CH3COCH2CH(OCH3)2 is reacted with hydrazine, two isomeric products are formed. Indicate their structures and the major product.arrow_forward
- Is it possible to obtain addition derivatives to nitrogen in position 2 of pyrazoles by reaction with electrophilic agents? Reason for this.arrow_forwardStarting from 1,3-dicarbonyl derivatives to obtain isooxazoles and isothiazoles. Indicate whether synthetic methods exist.arrow_forwardIn the synthesis of benzotriazole, adding NaNO2 heats the solution. State the reason.arrow_forward
 Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning





