
World of Chemistry, 3rd edition
3rd Edition
ISBN: 9781133109655
Author: Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher: Brooks / Cole / Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 1.2, Problem 1RQ
Interpretation Introduction
Interpretation: The difference between a qualitative observation and a quantitative observation needs to be explained.
Concept Introduction: Observations are essential in the subject chemistry. Chemists use observations to collect as well as record the data. Through observations, researchers develop and test hypotheses & theories.
Expert Solution & Answer
Answer to Problem 1RQ
Qualitative observation is generally conducted on a small data
Explanation of Solution
The difference between a qualitative observation and a quantitative observationis mentioned below:
| Qualitative observation | Quantitative observation |
| Qualitative observations use a person’s sense to observe the result. | Quantitative observations are observed with instruments (ruler, beakers, etc.) |
| Qualitative observations are variable. | Quantitative observations are invariable. |
| Qualitative observations are not accurate. | Quantitative observations are accurate. |
| Qualitative observations are not measurable. | Quantitative observations are measurable. |
| Qualitative observations cannot be noted in expressions of numbers. | Quantitative observations can be noted in expressions of numbers. |
For example: Freezing point of 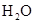 is more polar than its boiling point. is more polar than its boiling point. | For example: The freezing point of 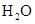 is is 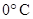 The boiling point of The boiling point of 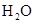 is is 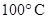 |
Chapter 1 Solutions
World of Chemistry, 3rd edition
Ch. 1.1 - Prob. 1RQCh. 1.1 - Prob. 2RQCh. 1.1 - Prob. 3RQCh. 1.1 - Prob. 4RQCh. 1.1 - Prob. 5RQCh. 1.1 - Prob. 6RQCh. 1.2 - Prob. 1RQCh. 1.2 - Prob. 2RQCh. 1.2 - Prob. 3RQCh. 1.2 - Prob. 4RQ
Ch. 1.3 - Prob. 1RQCh. 1.3 - Prob. 2RQCh. 1.3 - Prob. 3RQCh. 1.3 - Prob. 4RQCh. 1 - Prob. 1ACh. 1 - Prob. 2ACh. 1 - Prob. 3ACh. 1 - Prob. 4ACh. 1 - Prob. 5ACh. 1 - Prob. 6ACh. 1 - Prob. 7ACh. 1 - Prob. 8ACh. 1 - Prob. 9ACh. 1 - Prob. 10ACh. 1 - Prob. 11ACh. 1 - Prob. 12ACh. 1 - Prob. 13ACh. 1 - Prob. 14ACh. 1 - Prob. 15ACh. 1 - Prob. 16ACh. 1 - Prob. 17ACh. 1 - Prob. 18ACh. 1 - Prob. 19ACh. 1 - Prob. 1STPCh. 1 - Prob. 2STPCh. 1 - Prob. 3STPCh. 1 - Prob. 4STPCh. 1 - Prob. 5STPCh. 1 - Prob. 6STPCh. 1 - Prob. 7STPCh. 1 - Prob. 8STPCh. 1 - Prob. 9STPCh. 1 - Prob. 10STP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the mechanism for this?arrow_forward21.50 Determine the combinations of haloalkane(s) and alkoxide(s) that could be used to synthesize the following ethers through Williamson ether synthesis. (a) (c) (d) (e) (f) H₂COarrow_forward1. Arrange the following in order of increasing bond energy (lowest bond energy first, highest bond energy last). Provide your rationale. C=C, C-F, C=C, C-N, C-C List the bond order for each example.arrow_forward
- What is the major enolate formed when treated with LDA? And why that one?arrow_forward4. Calculate the total number of sigma bonds and total number of pi bonds in each of the following compounds. a. HH :D: +1 I H-N-C-C-O-H I H b. HH H Н :N=C-C-C=C-CEC-H :0: total o H-C-H H-C = `C-H I H. 11 H-C = C= CH H total o total π total π 1 Harrow_forwardIn the following reaction, what quantity in moles of CH₃OH are required to give off 4111 kJ of heat? 2 CH₃OH (l) + 3 O₂ (g) → 2 CO₂ (g) + 4 H₂O(g) ∆H° = -1280. kJarrow_forward
- Indicate the processes in the dismutation of Cu2O.arrow_forward1. Consider these three reactions as the elementary steps in the mechanism for a chemical reaction. 2600 2400 2200 2000 1800 1600 1400 1200 1000 800 Potential Energy (kJ) 600 400 200 0 -200- -400 -600- -800 (i) Cl₂ (g) + Pt(s) → 2Cl (g) + Pt(s) (ii) Cl (g)+ CO (g) + Pt (s) → CICO (g) + Pt (s) Ea = 1550 kJ Ea = 2240 kJ (iii) Cl (g) + CICO (g) → Cl₂CO (g) Ea = 2350 kJ AH=-950 kJ ΔΗ = 575 ΚΙ AH=-825 kJ a. Draw the potential energy diagram for the reaction. Label the data points for clarity. The potential energy of the reactants is 600 kJ Reaction Progress b. What is the overall chemical equation? c. What is the overall change in enthalpy for the above chemical reaction? d. What is the overall amount of activation energy for the above chemical reaction? e. Which reaction intermediate would be considered a catalyst (if any) and why? f. If you were to add 2700kJ of energy to the reaction (e.g. 2700 kl of heat or electricity), would you be able to make the reaction reverse itself (i.e. have…arrow_forwarddraw the enolate anion and the carbonyl that would be needed to make this product through an aldol addition reaction.arrow_forward
- Draw the Michael Adduct and the final product of the Robinson annulation reaction. Ignore inorganic byproducts.arrow_forwardDraw the Michael adduct and final product of the Robinson annulation reaction. Ignore inorganic byproductsarrow_forwardPost Lab Questions. 1) Draw the mechanism of your Diels-Alder cycloaddition. 2) Only one isomer of product is formed in the Diels-Alder cycloaddition. Why? 3) Imagine that you used isoprene as diene - in that case you don't have to worry about assigning endo vs exo. Draw the "endo" and "exo" products of the Diels-Alder reaction between isoprene and maleic anhydride, and explain why the distinction is irrelevant here. 4) This does not hold for other dienes. Draw the exo and endo products of the reaction of cyclohexadiene with maleic anhydride. Make sure you label your answers properly as endo or exo. 100 °C Xylenes ??? 5) Calculate the process mass intensity for your specific reaction (make sure to use your actual amounts of reagent).arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
The Creation of Chemistry - The Fundamental Laws: Crash Course Chemistry #3; Author: Crash Course;https://www.youtube.com/watch?v=QiiyvzZBKT8;License: Standard YouTube License, CC-BY