
Concept explainers
12-27 Explain why each name is incorrect and then write a correct name.
- 2-Ethyl-l-propene
- 5-lsopropylcyclohexene
- 4-Methyl-4-hexene
- 2-sec-Butyl-l-butene
- 6,6-Dimethylcyclohexene
- 2-Ethyl-2-hexene
(a)
Interpretation:
To identify the error in name of the given compound and write the correct IUPAC names.
Concept Introduction:
The rules to write the IUPAC names are as follows-
- The longest carbon chain is identified, and the root name is given accordingly.
- All the substituents attached to the root chain are determined.
- The position of the substituents is so assigned that the sum of their positions comes to be the least of all possible positions.
- The prefix di, tri, tetra is used if the same substituent is present two, three and four times respectively.
- All the substituents in the name are written in alphabetical order.
- For a cyclic hydrocarbon the prefix cyclo- is used.
Answer to Problem 17P
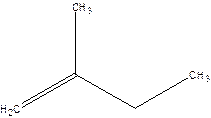
2-Methyl-but-1-ene.
Explanation of Solution
2-Ethyl-1-propene.
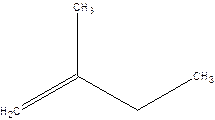
Error- longest chain not correctly located.
Correct name-
2-Methyl-but-1-ene.
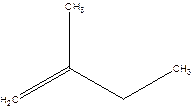
(b)
Interpretation:
To identify the error in name of the given compound and write the correct IUPAC names.
Concept Introduction:
The rules to write the IUPAC names are as follows-
- The longest carbon chain is identified, and the root name is given accordingly.
- All the substituents attached to the root chain are determined.
- The position of the substituents is so assigned that the sum of their positions comes to be the least of all possible positions.
- The prefix di, tri, tetra is used if the same substituent is present two, three and four times respectively.
- All the substituents in the name are written in alphabetical order.
- For a cyclic hydrocarbon the prefix cyclo- is used.
Answer to Problem 17P
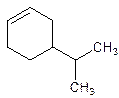
4-Isopropylcyclohexene.
Explanation of Solution
5-Isopropylcyclohexene.
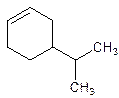
Error- position of the substituent not correctly mentioned.
Correct name-
4-Isopropylcyclohexene.
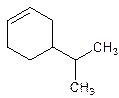
(c)
Interpretation:
To identify the error in name of the given compound and write the correct IUPAC names.
Concept Introduction:
The rules to write the IUPAC names are as follows-
- The longest carbon chain is identified, and the root name is given accordingly.
- All the substituents attached to the root chain are determined.
- The position of the substituents is so assigned that the sum of their positions comes to be the least of all possible positions.
- The prefix di, tri, tetra is used if the same substituent is present two, three and four times respectively.
- All the substituents in the name are written in alphabetical order.
- For a cyclic hydrocarbon the prefix cyclo- is used.
Answer to Problem 17P

3-Methyl-2-hexene.
Explanation of Solution
4-Methyl-4-hexene.
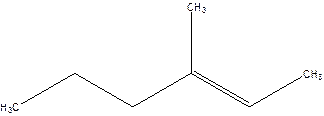
Error- position of the double bond not correctly mentioned.
Correct name-
3-Methyl-2-hexene.
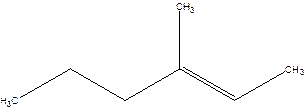
(d)
Interpretation:
To identify the error in name of the given compound and write the correct IUPAC names.
Concept Introduction:
The rules to write the IUPAC names are as follows-
- The longest carbon chain is identified, and the root name is given accordingly.
- All the substituents attached to the root chain are determined.
- The position of the substituents is so assigned that the sum of their positions comes to be the least of all possible positions.
- The prefix di, tri, tetra is used if the same substituent is present two, three and four times respectively.
- All the substituents in the name are written in alphabetical order.
- For a cyclic hydrocarbon the prefix cyclo- is used.
Answer to Problem 17P
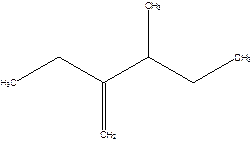
2-Ethyl-3-methyl -1-pentene.
Explanation of Solution
2-sec-butyl-1-butene.
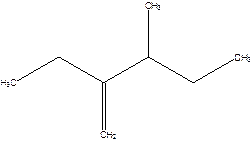
Error- longest chain not correctly determined.
Correct name-
2-Ethyl-3-methyl -1-pentene.
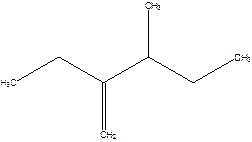
(e)
Interpretation:
To identify the error in name of the given compound and write the correct IUPAC names.
Concept Introduction:
The rules to write the IUPAC names are as follows-
- The longest carbon chain is identified, and the root name is given accordingly.
- All the substituents attached to the root chain are determined.
- The position of the substituents is so assigned that the sum of their positions comes to be the least of all possible positions.
- The prefix di, tri, tetra is used if the same substituent is present two, three and four times respectively.
- All the substituents in the name are written in alphabetical order.
- For a cyclic hydrocarbon the prefix cyclo- is used.
Answer to Problem 17P
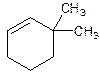
3,3-Dimethylcyclohexene.
Explanation of Solution
6,6-Dimethylcyclohexene.
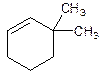
Error- position of substituents not correctly determined.
Correct name-
3,3-Dimethylcyclohexene.
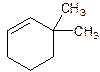
(f)
Interpretation:
To identify the error in name of the given compound and write the correct IUPAC names.
Concept Introduction:
The rules to write the IUPAC names are as follows-
- The longest carbon chain is identified, and the root name is given accordingly.
- All the substituents attached to the root chain are determined.
- The position of the substituents is so assigned that the sum of their positions comes to be the least of all possible positions.
- The prefix di, tri, tetra is used if the same substituent is present two, three and four times respectively.
- All the substituents in the name are written in alphabetical order.
- For a cyclic hydrocarbon the prefix cyclo- is used.
Answer to Problem 17P

3-Methyl-3-Heptene.
Explanation of Solution
2-Ethyl-2-hexene.

Error- longest chain not correctly determined.
Correct name-
3-Methyl-3-Heptene.

Want to see more full solutions like this?
Chapter 12 Solutions
Introduction to General, Organic and Biochemistry
- Draw and name the R groups of all 20 amino acids.arrow_forward3. Two solutions are prepared using the same solute: Solution A: 0.14 g of the solute dissolves in 15.4 g of t-butanol Solution B: 0.17 g of the solute dissolves in 12.7 g of cyclohexane Which solution has the greatest freezing point change? Show calculations and explain.arrow_forward2. Give the ground state electron configuration (e.g., 02s² σ*2s² П 2p²) for these molecules and deduce its bond order. Ground State Configuration Bond Order H2+ 02- N2arrow_forward
- 1. This experiment is more about understanding the colligative properties of a solution rather than the determination of the molar mass of a solid. a. Define colligative properties. b. Which of the following solutes has the greatest effect on the colligative properties for a given mass of pure water? Explain. (i) 0.01 mol of CaCl2 (ii) 0.01 mol of KNO3 (iii) 0.01 mol of CO(NH2)2 (an electrolyte) (an electrolyte) (a nonelectrolyte)arrow_forward5. b. For Trials 2 and 3, the molar mass of the solute was 151 g/mol and 143 g/mol respectively. a. What is the average molar mass of the solute ? b. What are the standard deviation and the relative standard deviation (%RSD) for the molar mass of the solute ?arrow_forwardShow work. Don't give Ai generated solutionarrow_forward
- 2. Explain why ice cubes formed from water of a glacier freeze at a higher temperature than ice cubes formed from water of an under- ground aquifer. Photodynamic/iStockphotoarrow_forwardShow reaction mechanism. don't give Ai generated solutionarrow_forward7. Draw the Lewis structures and molecular orbital diagrams for CO and NO. What are their bond orders? Are the molecular orbital diagrams similar to their Lewis structures? Explain. CO Lewis Structure NO Lewis Structure CO Bond Order NO Bond Order NO Molecular Orbital Diagram CO Molecular Orbital Diagramarrow_forward
- 5. The existence of compounds of the noble gases was once a great surprise and stimulated a great deal of theoretical work. Label the molecular orbital diagram for XeF (include atom chemical symbol, atomic orbitals, and molecular orbitals) and deduce its ground state electron configuration. Is XeF likely to have a shorter bond length than XeF+? Bond Order XeF XeF+arrow_forward6. Draw the molecular orbital diagram shown to determine which of the following is paramagnetic. B22+ B22+, B2, C22, B22 and N22+ Molecular Orbital Diagram B2 C22- B22- N22+ Which molecule is paramagnetic?arrow_forward3. Put the following species in order of increasing bond length by using molecular orbital diagrams and calculating their bond orders: F2, F2, F2+ Molecular Orbital Diagram F2 F2 F2+ Bond Order Shortest bond: Longest bondarrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning




