
Concept explainers
(a)
Interpretation:
To analyze whether the given statement- There are two classes of
Concept Introduction:
Hydrocarbons consists of carbon atoms and hydrogen atoms. They are further classified as saturated or unsaturated on the basis that the hydrocarbon has all single bonds, or it involves double or triple bond also.
The hydrocarbon that has all single bond is called as
The hydrocarbon that has at least one double bond is called as alkene wit the general formula
The hydrocarbon that has at least one triple bond is called as alkyne wit the general formula
(a)
Answer to Problem 1P
There are two classes of unsaturated hydrocarbons-alkenes and alkynes. Thus, the statement is true.
Explanation of Solution
Hydrocarbons consists of carbon atoms and hydrogen atoms. They are further classified as saturated or unsaturated on the basis that the hydrocarbon has all single bonds or it involves double or triple bond also.
The hydrocarbon that has all single bond is called as alkane with the general formula
Example- Ethane

The hydrocarbon that has at least one double bond is called as alkene with the general formula
Example- Ethene
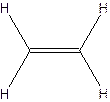
The hydrocarbon that has at least one triple bond is called as alkyne with the general formula
Example- Ethyene

Since all the bonds in alkenes and alkynes are not single bonds, they are unsaturated hydrocarbons
(b)
Interpretation:
To analyze whether the given statement- The bulk of the ethylene used by the chemical industry worldwide is obtained from renewable resources , is true or false.
Concept Introduction:
Ethylene is an unsaturated hydrocarbon, with the formula
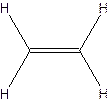
Renewable sources are those sources which when used once can be generated again.e.g. solar energy is a renewable source of energy.
Non-renewable sourcesare those sources which when used once are generated after millions of years e.g. coal and petroleum are non-renewable sources of energy.
The bulk of the ethylene used by the chemical industry worldwide is obtained from non-renewable resources. Thus, the statement is true.
There are several methods by which ethylene is extracted
- Steam Cracking of-
- Ethane and propane from natural gas or crude oil
- Naptha from crude oil
- Catalytic cracking of gas oil from crude oil
Crude oil and natural gas both are non-renewable sources. Therefore, the bulk of the ethylene used by the chemical industry worldwide is obtained from non-renewable resources
(c)
Interpretation:
To analyze whether the give statement- Ethylene and acetylene are constitutional isomers, is true or false.
Concept Introduction:
There are compounds which have same constituents but differ in the arrangement of atoms in space. The constitutional isomers are those compounds that have same molecular formula but different structural formula. For example- 2-Chloro butane and 1-Chloro butane are constitutional isomers.
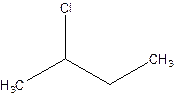
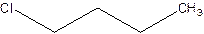
2-Chlorobutane 1-Chlorobutane
Ethylene and acetylene are not constitutional isomers. Thus, the statement is false.
There are compounds which have same constituents but differ in the arrangement of atoms in space. The constitutional isomers are those compounds that have same molecular formula but different structural formula.
The molecular formula for ethylene
The structural formula for ethylene-
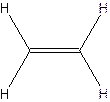
The molecular formula for acetylene
The structural formula for acetylene-
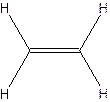
Since ethylene and acetylene neither have same molecular formula nor structural formula. So they are not constitutional formula.
(d)
Interpretation:
To analyze whether the give statement- Cyclohexane and 1-hexene are constitutional isomers, is true or false.
Concept Introduction:
There are compounds which have same constituents but differ in the arrangement of atoms in space. The constitutional isomers are those compounds that have same molecular formula but different structural formula. For example- 2-Chloro butane and 1-Chloro butane are constitutional isomers.
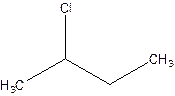
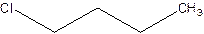
2-Chlorobutane 1-Chlorobutane
Cyclohexane and 1-hexene are constitutional isomers. Thus, the statement is true.
There are compounds which have same constituents but differ in the arrangement of atoms in space. The constitutional isomers are those compounds that have same molecular formula but different structural formula.
The molecular formula for Cyclohexane
The structural formula for Cyclohexane -
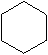
Cyclohexane
The molecular formula for 1-hexene
The structural formula for 1-hexene -

1-hexene
Since, cyclohexane and 1-hexene have same molecular formula and structural formula so, they are constitutional formula.
(b)
Answer to Problem 1P
The bulk of the ethylene used by the chemical industry worldwide is obtained from non-renewable resources. Thus, the statement is true.
Explanation of Solution
There are several methods by which ethylene is extracted
- Steam Cracking of-
- Ethane and propane from natural gas or crude oil
- Naptha from crude oil
- Catalytic cracking of gas oil from crude oil
Crude oil and natural gas both are non-renewable sources. Therefore, the bulk of the ethylene used by the chemical industry worldwide is obtained from non-renewable resources
(c)
Interpretation:
To analyze whether the give statement- Ethylene and acetylene are constitutional isomers, is true or false.
Concept Introduction:
There are compounds which have same constituents but differ in the arrangement of atoms in space. The constitutional isomers are those compounds that have same molecular formula but different structural formula. For example- 2-Chloro butane and 1-Chloro butane are constitutional isomers.

2-Chlorobutane 1-Chlorobutane
(c)
Answer to Problem 1P
Ethylene and acetylene are not constitutional isomers. Thus, the statement is false.
Explanation of Solution
There are compounds which have same constituents but differ in the arrangement of atoms in space. The constitutional isomers are those compounds that have same molecular formula but different structural formula.
The molecular formula for ethylene
The structural formula for ethylene-
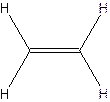
The molecular formula for acetylene
The structural formula for acetylene-
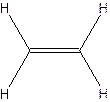
Since ethylene and acetylene neither have same molecular formula nor structural formula. So they are not constitutional formula.
(d)
Interpretation:
To analyze whether the give statement- Cyclohexane and 1-hexene are constitutional isomers, is true or false.
Concept Introduction:
There are compounds which have same constituents but differ in the arrangement of atoms in space. The constitutional isomers are those compounds that have same molecular formula but different structural formula. For example- 2-Chloro butane and 1-Chloro butane are constitutional isomers.
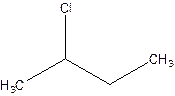

2-Chlorobutane 1-Chlorobutane
(d)
Answer to Problem 1P
Cyclohexane and 1-hexene are constitutional isomers. Thus, the statement is true.
Explanation of Solution
There are compounds which have same constituents but differ in the arrangement of atoms in space. The constitutional isomers are those compounds that have same molecular formula but different structural formula.
The molecular formula for Cyclohexane
The structural formula for Cyclohexane -

Cyclohexane
The molecular formula for 1-hexene
The structural formula for 1-hexene -

1-hexene
Since, cyclohexane and 1-hexene have same molecular formula and structural formula so, they are constitutional formula.
Want to see more full solutions like this?
Chapter 12 Solutions
Introduction to General, Organic and Biochemistry
- :0: :0: Select to Add Arrows :0: (CH3)2NH :0: ■ Select to Add Arrows :0: :0: (CH3)2NH ■ Select to Add Arrowsarrow_forwardDraw the product of the following H action sequence. Ignore any inorganic byproducts formed. 1. (CH3CH2)2CuLi, THF 2. CH3Br Q Atoms, Bonds and Rings H Charges ㅁarrow_forwardPlease help me with this the problem is so confusingarrow_forward
- 14 Question (1 point) Disiamylborane adds to a triple bond to give an alkenylborane. Upon oxidation with OH, H2O2, the alkenylborane will form an enol that tautomerizes to an aldehyde. In the first box below, draw the mechanism arrows for the reaction of disiamylborane with the alkyne, and in the last box draw the structure of the aldehyde. 4th attempt Feedback i > 3rd attempt OH, H2O2 i See Periodic Table See Hintarrow_forwardanswer with mechanisms and steps. handwritten please!arrow_forwardHello I need some help with Smartwork. For drawing structure B, I know the correct answer is CH₃B₂, but when I try to type it in, it keeps giving me CH₄BH₃ instead. Do you know how I should write it properly? Should I use a bond or something else?arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,





