
Interpretation:
The reagent used to accomplish the given transformation has to be identified.
Concept Introduction:
Radical Bromination is a process in which the bromine atom is obtained as radical due to irradiation and this attack the carbon atom to form the brominated product. The bromine atom gets substituted in the more substituted carbon atom.
Elimination reactions are the one in which the groups are lost and the saturated bonds are converted to unsaturated bonds. Usually the substitution reaction compete with elimination reaction.
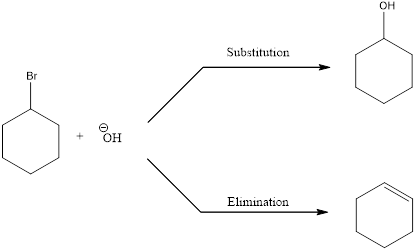
In elimination reaction, the beta proton is removed together with the leaving group to form a double bond.
E2 reaction proceeds through a single step without any formation of intermediates. The base abstracts a proton form the substrate and the loss of leaving group also happens resulting in the formation of double bond. E2 stands for bimolecular elimination.
The proton can be abstracted by the base in two different ways leading to a regiochemical outcome. In the given reaction below, two
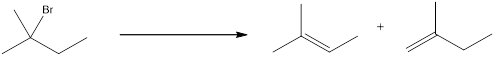
The two alkenes that are formed can be given different names. If the alkene is more substituted means it is known as Zaitsev product, and if the alkene formed is less substituted means it is known as Hofmann product. Generally more substituted product is the major product (Zaitsev product).
If the reaction is performed with a sterically hindered base, the major product will be less substituted alkene (Hofmann product). The regiochemical outcome can be decided by choosing the base. Some of the sterically hindered bases are,

Addition reactions are the one in which the groups are added to an unsaturated double bond. The double bond is destroyed because the groups are added across the double bond. If the considered alkene is a symmetrical one, then there is no difference in the product obtained but if the alkene is an unsymmetrical one, then regiochemistry decides the products that are obtained.
When two similar groups are added across a double bond, regiochemistry is irrelevant. When adding two different groups across the double bond of an unsymmetrical alkene, the regiochemistry becomes relevant. In simple words, it can be said that, when two different groups are added across a double bond, then regiochemistry becomes relevant.
Markovnikov Addition
In the vinylic bond, if the bulky group gets substituted in the carbon atom that is more substituted means, it is known as Markovnikov’s addition.

anti-Markovnikov Addition
In the vinylic bond, if the bulky group gets substituted in the carbon atom that is less substituted means, it is known as anti-Markovnikov’s addition.
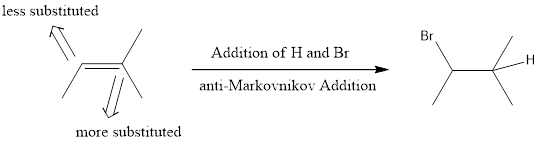
If hydrogen halide is used in presence of hydrogen peroxide, anti-Markovnikov addition takes place. This means the halide ends up on less substituted carbon atom. This is because when peroxide is used the mechanism that the reaction undergoes is radical mechanism. This involves a radical intermediate instead of ionic intermediate. The main difference between ionic mechanism and radical mechanism is that proton is installed first in ionic mechanism while in radical mechanism, halide is installed first. This is the reason why ionic mechanism gives Markovnikov product and radical mechanism gives anti-Markovnikov product.

Want to see the full answer?
Check out a sample textbook solution
Chapter 11 Solutions
ORGANIC CHEMISTRY-NEXTGEN+BOX (1 SEM.)
- Determine if the following salt is neutral, acidic or basic. If acidic or basic, write the appropriate equilibrium equation for the acid or base that exists when the salt is dissolved in aqueous solution. If neutral, simply write only NR. Be sure to include the proper phases for all species within the reaction LiNO3arrow_forwardAn unknown weak acid with a concentration of 0.410 M has a pH of 5.600. What is the Ka of the weak acid?arrow_forward(racemic) 19.84 Using your reaction roadmaps as a guide, show how to convert 2-oxepanone and ethanol into 1-cyclopentenecarbaldehyde. You must use 2-oxepanone as the source of all carbon atoms in the target molecule. Show all reagents and all molecules synthesized along the way. & + EtOH H 2-Oxepanone 1-Cyclopentenecarbaldehydearrow_forward
- R₂ R₁ R₁ a R Rg Nu R₂ Rg R₁ R R₁₂ R3 R R Nu enolate forming R₁ R B-Alkylated carbonyl species or amines Cyclic B-Ketoester R₁₁ HOB R R₁B R R₁₂ B-Hydroxy carbonyl R diester R2 R3 R₁ RB OR R₂ 0 aB-Unsaturated carbonyl NaOR Aldol HOR reaction 1) LDA 2) R-X 3) H₂O/H₂O ketone, aldehyde 1) 2°-amine 2) acid chloride 3) H₂O'/H₂O 0 O R₁ R₁ R R₁ R₁₂ Alkylated a-carbon R₁ H.C R₁ H.C Alkylated methyl ketone acetoacetic ester B-Ketoester ester R₁ HO R₂ R B-Dicarbonyl HO Alkylated carboxylic acid malonic ester Write the reagents required to bring about each reaction next to the arrows shown. Next, record any regiochemistry or stereochemistry considerations relevant to the reaction. You should also record any key aspects of the mechanism, such as forma- tion of an important intermediate, as a helpful reminder. You may want to keep track of all reactions that make carbon-carbon bonds, because these help you build large molecules from smaller fragments. This especially applies to the reactions in…arrow_forwardProvide the reasonable steps to achieve the following synthesis.arrow_forwardIdentify which compound is more acidic. Justify your choice.arrow_forward
- Provide the reasonable steps to achieve the following synthesis.arrow_forwardWhen anisole is treated with excess bromine, the reaction gives a product which shows two singlets in 1H NMR. Draw the product.arrow_forward(ii) Draw a reasonable mechanism for the following reaction: CI NaOH heat OH (hint: SNAr Reaction) :arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





