
Interpretation:
The reagent that can accomplish the given reaction has to be identified.
Concept Introduction:
Addition reactions are the one in which the groups are added to an unsaturated double bond. The double bond is destroyed because the groups are added across the double bond. If the considered
When two similar groups are added across a double bond, regiochemistry is irrelevant. When adding two different groups across the double bond of an unsymmetrical alkene, the regiochemistry becomes relevant. In simple words, it can be said that, when two different groups are added across a double bond, then regiochemistry becomes relevant.
Markovnikov Addition
In the vinylic bond, if the bulky group gets substituted in the carbon atom that is more substituted means, it is known as Markovnikov’s addition.

anti-Markovnikov Addition
In the vinylic bond, if the bulky group gets substituted in the carbon atom that is less substituted means, it is known as anti-Markovnikov’s addition.
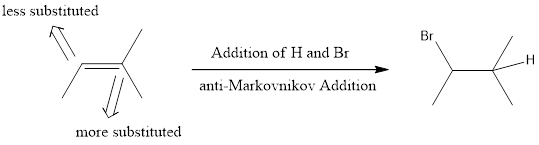
Want to see the full answer?
Check out a sample textbook solution
Chapter 11 Solutions
ORGANIC CHEMISTRY-NEXTGEN+BOX (1 SEM.)
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





