
Organic Chemistry (8th Edition)
8th Edition
ISBN: 9780134042282
Author: Paula Yurkanis Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 1.16, Problem 46P
What of the following molecules would you expect to have a dipole moment of zero? To answer parts g and h, you may need to review the Problem Solving Strategy on p. 39.
- a. CH3CH3

- b. CH2Cl2
- c. NH3
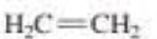

- d. BeCl2
- e. BF3
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
b. Please complete the zig-zag conformation of the compound
(3R,4S)-3,4-dichloro-2,5-dimethylhexane by writing the respective atoms in the boxes.
4
c. Serricornin, the female-produced sex pheromone of the cigarette beetle, has the following structure.
OH
What is the maximum number of possible stereoisomers?
Is this structure a meso compound?
d. Please consider the natural product alkaloids shown below.
Are these two structures enantiomers, diastereomers or conformers?
H
HO
H
H
HN
HO
HN
R
R
с
R=H
cinchonidine
R=ET
cinchonine
H
Nail polish remover containing acetone was spilled in a room 5.23 m × 3.28 m × 2.76 m.
Measurements indicated that 2,250 mg of acetone evaporated. Calculate the acetone concentration in micrograms per cubic meter.
Chapter 1 Solutions
Organic Chemistry (8th Edition)
Ch. 1.1 - Oxygen has three isotopes, 16O, 17O, and 18O. The...Ch. 1.1 - a. How many protons do the following species...Ch. 1.1 - Chlorine has two isotopes, 35Cl and 37Cl; 75.77%...Ch. 1.2 - Prob. 4PCh. 1.2 - a. Write the ground-state electronic configuration...Ch. 1.2 - Look at the relative positions of each pair of...Ch. 1.3 - a. Find potassium (K) in the periodic table and...Ch. 1.3 - Which bond is more polar? a. b. c. d.Ch. 1.3 - Which of the following has a. the most polar bond?...Ch. 1.3 - Use the symbols + and to show the direction of...
Ch. 1.3 - Explain why HCL has a smaller dipole moment than...Ch. 1.3 - After examining the potential maps for LiH, HF,...Ch. 1.4 - An atom with a formal charge does not necessarily...Ch. 1.4 - Prob. 16PCh. 1.4 - a. Draw two Lewis structure for C2H6O. b. Draw...Ch. 1.4 - Draw the lone-pair electrons that are not shown in...Ch. 1.4 - Prob. 20PCh. 1.4 - Which of the atoms in the molecular models in...Ch. 1.4 - Prob. 22PCh. 1.4 - Prob. 23PCh. 1.5 - Draw the following orbitals: a. 3s orbital b. 4s...Ch. 1.6 - Prob. 25PCh. 1.6 - Indicate the kind of molecular orbital (, , , or )...Ch. 1.7 - What orbitals are used to form the 10 sigma bonds...Ch. 1.7 - Explain why a bond formed by overlap of s orbital...Ch. 1.9 - Put n number in each of the blanks: a. __ s...Ch. 1.9 - For each of the given species: a. Draw its Lewis...Ch. 1.11 - Predict the approximate bond angles in a. the...Ch. 1.11 - According to the potential map for the ammonium...Ch. 1.12 - Prob. 35PCh. 1.13 - a. What are the relative lengths and strengths of...Ch. 1.13 - Prob. 38PCh. 1.14 - Describe the orbitals used in bonding and the bond...Ch. 1.15 - Which of the bond in a carbon-oxygen double bond...Ch. 1.15 - Would you expect a CC bond formed by sp2sp2...Ch. 1.15 - Caffeine is a natural insecticide found in the...Ch. 1.15 - a. What is the hybridization of each of the carbon...Ch. 1.15 - Predict the approximate bond angles for a. the CNC...Ch. 1.16 - What of the following molecules would you expect...Ch. 1.16 - Account for the difference in the shape and color...Ch. 1.16 - If the dipole moment of CH3F is 1.847 D and the...Ch. 1 - Draw a Lewis structure for each of the following:...Ch. 1 - Prob. 50PCh. 1 - What is the hybridization of all the atoms (other...Ch. 1 - Draw the condensed structure of a compound that...Ch. 1 - Predict the approximate bond angles: a. the CNH...Ch. 1 - Prob. 54PCh. 1 - Draw a Lewis structure for each of the following:...Ch. 1 - What is the hybridization of each of the carbon...Ch. 1 - Rank the bonds from most polar. a. CO, CF, CN b....Ch. 1 - Draw a Lewis structure for each of the following:...Ch. 1 - Prob. 59PCh. 1 - What is the hybridization of the indicated atom in...Ch. 1 - Predict the approximate bond angles for the...Ch. 1 - Prob. 62PCh. 1 - Draw the missing lone-pair electrons and assigns...Ch. 1 - a. Which of the indicated bonds in each molecule...Ch. 1 - For each of the following molecules, indicate the...Ch. 1 - Draw a Lewis structure for each of the following:...Ch. 1 - Prob. 67PCh. 1 - Rank the following compounds from highest dipole...Ch. 1 - In which orbitals are the lone pairs in nicotine?Ch. 1 - Prob. 70PCh. 1 - Prob. 71PCh. 1 - a. Which of the species have bond angles of 109.5?...Ch. 1 - Prob. 73PCh. 1 - Which compound has a larger dipole moment: CH3Cl...Ch. 1 - Prob. 75PCh. 1 - Explain why CH3Cl has a greater dipole moment than...Ch. 1 - a. Draw a Lewis structure for each of the...Ch. 1 - There are three isomers with molecular formula...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please help me answer number 1. 1. If your graphs revealed a mathematical relationship between specific heat and atomic mass, write down an equation for the relationship. I also don't understand, is the equation from the line regression the one that I'm suppose use to show the relationship? If so could you work it all the way out?arrow_forwardDescribe the principle of resonance and give a set of Lewis Structures to illustrate your explanation.arrow_forwardDon't used hand raitingarrow_forward
- It is not unexpected that the methoxyl substituent on a cyclohexane ring prefers to adopt the equatorial conformation. OMe H A G₂ = +0.6 kcal/mol OMe What is unexpected is that the closely related 2-methoxytetrahydropyran prefers the axial conformation: H H OMe OMe A Gp=-0.6 kcal/mol Methoxy: CH3O group Please be specific and clearly write the reason why this is observed. This effect that provides stabilization of the axial OCH 3 group in this molecule is called the anomeric effect. [Recall in the way of example, the staggered conformer of ethane is more stable than eclipsed owing to bonding MO interacting with anti-bonding MO...]arrow_forward206 Pb 82 Express your answers as integers. Enter your answers separated by a comma. ▸ View Available Hint(s) VAΣ ΜΕ ΑΣΦ Np, N₁ = 82,126 Submit Previous Answers ? protons, neutronsarrow_forwardPlease draw the inverted chair forms of the products for the two equilibrium reactions shown below. Circle the equilibrium reaction that would have a AG = 0, i.e., the relative energy of the reactant (to the left of the equilibrium arrows) equals the relative energy of the product? [No requirement to show or do calculations.] CH3 CH3 HH CH3 1 -CH3arrow_forward
- 5. Please consider the Newman projection of tartaric acid drawn below as an eclipsed conformer (1). Please draw the most stable conformer and two intermediate energy conformers noting that staggered conformers are lower in energy than eclipsed forms even if the staggered conformers have gauche relationships between groups. [Draw the substituents H and OH on the front carbons and H, OH and CO₂H on the back carbons based on staggered forms. -CO₂H is larger than -OH.] OH COH ICOOH COOH COOH 1 2 COOH COOH 3 4 Staggered Staggered Staggered (most stable) Indicate the number of each conformer above (1, 2, 3 and 4) that corresponds to the relative energies below. Ref=0 Rotation 6. (60 points) a. Are compounds 1 and 2 below enantiomers, diastereomers or identical? OH OH HO HO LOH HO HO OH 2 OH OH b. Please complete the zig-zag conformation of the compound (3R,4S)-3,4-dichloro-2,5-dimethylhexane by writing the respective atoms in the boxes. 3.arrow_forwardThe plutonium isotope with 144 neutrons Enter the chemical symbol of the isotope.arrow_forwardThe mass ratio of sodium to fluorine in sodium fluoride is 1.21:1. A sample of sodium fluoride produced 26.1 gg of sodium upon decomposition. How much fluorine was formed?arrow_forward
- 32S 16 Enter your answers numerically separated by a comma. Np. Nn = 跖 ΟΙ ΑΣΦ Submit Request Answer ? protons, neutronsarrow_forward2. Which dimethylcyclohexane compounds shown below exhibit symmetry and therefore are not chiral and would not rotate plane polarized light. 1 CH3 CH CH3 CH3 2 3 CH3arrow_forwardDon't used hand raitingarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Stoichiometry - Chemistry for Massive Creatures: Crash Course Chemistry #6; Author: Crash Course;https://www.youtube.com/watch?v=UL1jmJaUkaQ;License: Standard YouTube License, CC-BY
Bonding (Ionic, Covalent & Metallic) - GCSE Chemistry; Author: Science Shorts;https://www.youtube.com/watch?v=p9MA6Od-zBA;License: Standard YouTube License, CC-BY
General Chemistry 1A. Lecture 12. Two Theories of Bonding.; Author: UCI Open;https://www.youtube.com/watch?v=dLTlL9Z1bh0;License: CC-BY