
Organic Chemistry (8th Edition)
8th Edition
ISBN: 9780134042282
Author: Paula Yurkanis Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 1.6, Problem 26P
Indicate the kind of molecular orbital (σ, σ”, π, or π”) that results when the two atomic orbitals are combined:
a. 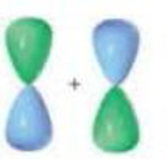
b. 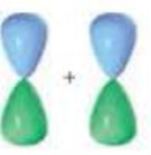
c. 
d. 
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Draw mechanism
Does Avogadro's number have units?
Explain why the total E in an Einstein depends on the frequency or wavelength of the light.
Chapter 1 Solutions
Organic Chemistry (8th Edition)
Ch. 1.1 - Oxygen has three isotopes, 16O, 17O, and 18O. The...Ch. 1.1 - a. How many protons do the following species...Ch. 1.1 - Chlorine has two isotopes, 35Cl and 37Cl; 75.77%...Ch. 1.2 - Prob. 4PCh. 1.2 - a. Write the ground-state electronic configuration...Ch. 1.2 - Look at the relative positions of each pair of...Ch. 1.3 - a. Find potassium (K) in the periodic table and...Ch. 1.3 - Which bond is more polar? a. b. c. d.Ch. 1.3 - Which of the following has a. the most polar bond?...Ch. 1.3 - Use the symbols + and to show the direction of...
Ch. 1.3 - Explain why HCL has a smaller dipole moment than...Ch. 1.3 - After examining the potential maps for LiH, HF,...Ch. 1.4 - An atom with a formal charge does not necessarily...Ch. 1.4 - Prob. 16PCh. 1.4 - a. Draw two Lewis structure for C2H6O. b. Draw...Ch. 1.4 - Draw the lone-pair electrons that are not shown in...Ch. 1.4 - Prob. 20PCh. 1.4 - Which of the atoms in the molecular models in...Ch. 1.4 - Prob. 22PCh. 1.4 - Prob. 23PCh. 1.5 - Draw the following orbitals: a. 3s orbital b. 4s...Ch. 1.6 - Prob. 25PCh. 1.6 - Indicate the kind of molecular orbital (, , , or )...Ch. 1.7 - What orbitals are used to form the 10 sigma bonds...Ch. 1.7 - Explain why a bond formed by overlap of s orbital...Ch. 1.9 - Put n number in each of the blanks: a. __ s...Ch. 1.9 - For each of the given species: a. Draw its Lewis...Ch. 1.11 - Predict the approximate bond angles in a. the...Ch. 1.11 - According to the potential map for the ammonium...Ch. 1.12 - Prob. 35PCh. 1.13 - a. What are the relative lengths and strengths of...Ch. 1.13 - Prob. 38PCh. 1.14 - Describe the orbitals used in bonding and the bond...Ch. 1.15 - Which of the bond in a carbon-oxygen double bond...Ch. 1.15 - Would you expect a CC bond formed by sp2sp2...Ch. 1.15 - Caffeine is a natural insecticide found in the...Ch. 1.15 - a. What is the hybridization of each of the carbon...Ch. 1.15 - Predict the approximate bond angles for a. the CNC...Ch. 1.16 - What of the following molecules would you expect...Ch. 1.16 - Account for the difference in the shape and color...Ch. 1.16 - If the dipole moment of CH3F is 1.847 D and the...Ch. 1 - Draw a Lewis structure for each of the following:...Ch. 1 - Prob. 50PCh. 1 - What is the hybridization of all the atoms (other...Ch. 1 - Draw the condensed structure of a compound that...Ch. 1 - Predict the approximate bond angles: a. the CNH...Ch. 1 - Prob. 54PCh. 1 - Draw a Lewis structure for each of the following:...Ch. 1 - What is the hybridization of each of the carbon...Ch. 1 - Rank the bonds from most polar. a. CO, CF, CN b....Ch. 1 - Draw a Lewis structure for each of the following:...Ch. 1 - Prob. 59PCh. 1 - What is the hybridization of the indicated atom in...Ch. 1 - Predict the approximate bond angles for the...Ch. 1 - Prob. 62PCh. 1 - Draw the missing lone-pair electrons and assigns...Ch. 1 - a. Which of the indicated bonds in each molecule...Ch. 1 - For each of the following molecules, indicate the...Ch. 1 - Draw a Lewis structure for each of the following:...Ch. 1 - Prob. 67PCh. 1 - Rank the following compounds from highest dipole...Ch. 1 - In which orbitals are the lone pairs in nicotine?Ch. 1 - Prob. 70PCh. 1 - Prob. 71PCh. 1 - a. Which of the species have bond angles of 109.5?...Ch. 1 - Prob. 73PCh. 1 - Which compound has a larger dipole moment: CH3Cl...Ch. 1 - Prob. 75PCh. 1 - Explain why CH3Cl has a greater dipole moment than...Ch. 1 - a. Draw a Lewis structure for each of the...Ch. 1 - There are three isomers with molecular formula...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- If the dissociation energy of one mole of O2 is 5.17 eV, determine the wavelength that must be used to dissociate it with electromagnetic radiation. Indicate how many Einstein's of this radiation are needed to dissociate 1 liter of O2 at 25°C and 1 atm of pressure.Data: 1 eV = 96485 kJ mol-1; R = 0.082 atm L K-1; c = 2.998x108 m s-1; h = 6.626x10-34 J s; NA = 6.022x 1023 mol-1arrow_forwardIndicate the number of Einsteins that are equivalent to 550 kJ mol⁻¹ of absorbed energy (wavelength 475 nm).arrow_forwardIndicate the number of einsteins that are equivalent to 550 kJ mol⁻¹ of absorbed energy?arrow_forward
- A unit used in photochemistry is the einstein. If 400 kJ mol-1 of energy has been absorbed, how many einsteins is this equivalent to?arrow_forwardFor the condensation reaction between Alanine and histidine write the amididation reaction mechanism using arrows then write the three letter code for the product of the reaction and the one letter code for the product of the reaction.arrow_forwardWrite the amididation reaction mechanism of p-aminophenol and acetic acid to produce acetaminophen please use arrows.arrow_forward
- Write the amididation reaction mechanism of a-aminophenol and acetic acid to produce acetaminophenarrow_forwardFor the condensation reaction between Alamine and histamine, please help me write the amididation reaction mechanism. Then write the three letter code for the product of the reaction, then write the one letter code for the product of the reaction. arrow_forwardHow to draw the reaction mechasnism belowarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax


Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning
Stoichiometry - Chemistry for Massive Creatures: Crash Course Chemistry #6; Author: Crash Course;https://www.youtube.com/watch?v=UL1jmJaUkaQ;License: Standard YouTube License, CC-BY
Bonding (Ionic, Covalent & Metallic) - GCSE Chemistry; Author: Science Shorts;https://www.youtube.com/watch?v=p9MA6Od-zBA;License: Standard YouTube License, CC-BY
General Chemistry 1A. Lecture 12. Two Theories of Bonding.; Author: UCI Open;https://www.youtube.com/watch?v=dLTlL9Z1bh0;License: CC-BY