
Organic Chemistry (8th Edition)
8th Edition
ISBN: 9780134042282
Author: Paula Yurkanis Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 1.15, Problem 43P
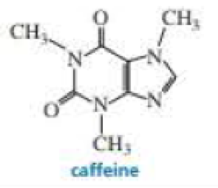
Caffeine is a natural insecticide found in the seeds and leaves of certain plants, where it kills insects that feed on the plant. Caffeine is extracted for human consumption from beans of the coffee plant, from Kola nuts, and from the leaves of tea plants. Because it stimulates the central nervous system, it temporarily prevents drowsiness. Add caffeine’s missing lone pairs to its structure.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Using the following two half-reactions, determine the pH range in which $NO_2^-\ (aq)$ cannot be found as the predominant chemical species in water.* $NO_3^-(aq)+10H^+(aq)+8e^-\rightarrow NH_4^+(aq)+3H_2O(l),\ pE^{\circ}=14.88$* $NO_2^-(aq)+8H^+(aq)+6e^-\rightarrow NH_4^+(aq)+2H_2O(l),\ pE^{\circ}=15.08$
Indicate characteristics of oxodec acid.
What is the final product when hexanedioic acid reacts with 1º PCl5 and 2º NH3.
Chapter 1 Solutions
Organic Chemistry (8th Edition)
Ch. 1.1 - Oxygen has three isotopes, 16O, 17O, and 18O. The...Ch. 1.1 - a. How many protons do the following species...Ch. 1.1 - Chlorine has two isotopes, 35Cl and 37Cl; 75.77%...Ch. 1.2 - Prob. 4PCh. 1.2 - a. Write the ground-state electronic configuration...Ch. 1.2 - Look at the relative positions of each pair of...Ch. 1.3 - a. Find potassium (K) in the periodic table and...Ch. 1.3 - Which bond is more polar? a. b. c. d.Ch. 1.3 - Which of the following has a. the most polar bond?...Ch. 1.3 - Use the symbols + and to show the direction of...
Ch. 1.3 - Explain why HCL has a smaller dipole moment than...Ch. 1.3 - After examining the potential maps for LiH, HF,...Ch. 1.4 - An atom with a formal charge does not necessarily...Ch. 1.4 - Prob. 16PCh. 1.4 - a. Draw two Lewis structure for C2H6O. b. Draw...Ch. 1.4 - Draw the lone-pair electrons that are not shown in...Ch. 1.4 - Prob. 20PCh. 1.4 - Which of the atoms in the molecular models in...Ch. 1.4 - Prob. 22PCh. 1.4 - Prob. 23PCh. 1.5 - Draw the following orbitals: a. 3s orbital b. 4s...Ch. 1.6 - Prob. 25PCh. 1.6 - Indicate the kind of molecular orbital (, , , or )...Ch. 1.7 - What orbitals are used to form the 10 sigma bonds...Ch. 1.7 - Explain why a bond formed by overlap of s orbital...Ch. 1.9 - Put n number in each of the blanks: a. __ s...Ch. 1.9 - For each of the given species: a. Draw its Lewis...Ch. 1.11 - Predict the approximate bond angles in a. the...Ch. 1.11 - According to the potential map for the ammonium...Ch. 1.12 - Prob. 35PCh. 1.13 - a. What are the relative lengths and strengths of...Ch. 1.13 - Prob. 38PCh. 1.14 - Describe the orbitals used in bonding and the bond...Ch. 1.15 - Which of the bond in a carbon-oxygen double bond...Ch. 1.15 - Would you expect a CC bond formed by sp2sp2...Ch. 1.15 - Caffeine is a natural insecticide found in the...Ch. 1.15 - a. What is the hybridization of each of the carbon...Ch. 1.15 - Predict the approximate bond angles for a. the CNC...Ch. 1.16 - What of the following molecules would you expect...Ch. 1.16 - Account for the difference in the shape and color...Ch. 1.16 - If the dipole moment of CH3F is 1.847 D and the...Ch. 1 - Draw a Lewis structure for each of the following:...Ch. 1 - Prob. 50PCh. 1 - What is the hybridization of all the atoms (other...Ch. 1 - Draw the condensed structure of a compound that...Ch. 1 - Predict the approximate bond angles: a. the CNH...Ch. 1 - Prob. 54PCh. 1 - Draw a Lewis structure for each of the following:...Ch. 1 - What is the hybridization of each of the carbon...Ch. 1 - Rank the bonds from most polar. a. CO, CF, CN b....Ch. 1 - Draw a Lewis structure for each of the following:...Ch. 1 - Prob. 59PCh. 1 - What is the hybridization of the indicated atom in...Ch. 1 - Predict the approximate bond angles for the...Ch. 1 - Prob. 62PCh. 1 - Draw the missing lone-pair electrons and assigns...Ch. 1 - a. Which of the indicated bonds in each molecule...Ch. 1 - For each of the following molecules, indicate the...Ch. 1 - Draw a Lewis structure for each of the following:...Ch. 1 - Prob. 67PCh. 1 - Rank the following compounds from highest dipole...Ch. 1 - In which orbitals are the lone pairs in nicotine?Ch. 1 - Prob. 70PCh. 1 - Prob. 71PCh. 1 - a. Which of the species have bond angles of 109.5?...Ch. 1 - Prob. 73PCh. 1 - Which compound has a larger dipole moment: CH3Cl...Ch. 1 - Prob. 75PCh. 1 - Explain why CH3Cl has a greater dipole moment than...Ch. 1 - a. Draw a Lewis structure for each of the...Ch. 1 - There are three isomers with molecular formula...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the final product when D-galactose reacts with hydroxylamine?arrow_forwardIndicate the formula of the product obtained by reacting methyl 5-chloro-5-oxopentanoate with 1 mole of 4-penten-1-ylmagnesium bromide.arrow_forwardIn the two chair conformations of glucose, the most stable is the one with all the OH groups in the equatorial position. Is this correct?arrow_forward
- please help me with my homeworkarrow_forwardhelparrow_forwardThe temperature on a sample of pure X held at 1.25 atm and -54. °C is increased until the sample boils. The temperature is then held constant and the pressure is decreased by 0.42 atm. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 2 0 0 200 400 temperature (K) Xarrow_forward
- QUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes *The values are all provided in the photo attached*arrow_forwardpressure (atm) 3 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 0 0 200 temperature (K) 400 аarrow_forwarder your payment details | bar xb Home | bartleby x + aleksogi/x/isl.exe/1o u-lgNskr7j8P3jH-1Qs_pBanHhviTCeeBZbufuBYT0Hz7m7D3ZcW81NC1d8Kzb4srFik1OUFhKMUXzhGpw7k1 O States of Matter Sketching a described thermodynamic change on a phase diagram 0/5 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 1 3- 0- 0 200 Explanation Check temperature (K) 400 X Q Search L G 2025 McGraw Hill LLC. All Rights Reserved Terms of Use Privacy Cearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning


Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning
Types of bonds; Author: Edspira;https://www.youtube.com/watch?v=Jj0V01Arebk;License: Standard YouTube License, CC-BY