
Concept explainers
For each compound. [1] Identify the
![Chapter 11.5, Problem 11.8PP, For each compound. [1] Identify the functional group; [2] draw out the complete compound, including , example 1](http://dev-ingestion-image-output.s3-website-us-east-1.amazonaws.com/9781259883989/Chapter-11/images/html_83989-11.5-11.8pp_image001.jpg)
b.
![Chapter 11.5, Problem 11.8PP, For each compound. [1] Identify the functional group; [2] draw out the complete compound, including , example 2](http://dev-ingestion-image-output.s3-website-us-east-1.amazonaws.com/9781259883989/Chapter-11/images/html_83989-11.5-11.8pp_image002.jpg)
d.
![Chapter 11.5, Problem 11.8PP, For each compound. [1] Identify the functional group; [2] draw out the complete compound, including , example 3](http://dev-ingestion-image-output.s3-website-us-east-1.amazonaws.com/9781259883989/Chapter-11/images/html_83989-11.5-11.8pp_image003.jpg)
(a)
Interpretation:
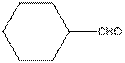
The functional group / groups should be identified and the complete structure with lone pairs on hetero-atoms of the following compound should be drawn:
Concept Introduction:
Organic molecules have some structural features in addition to
This table gives information about the compounds containing
| Type of Compound | General Structure |
| Aldehyde | 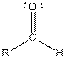 |
| Ketone | 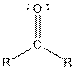 |
| Carboxylic acid | 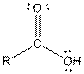 |
| Ester | 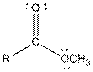 |
| Amide | 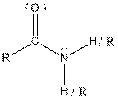 |
Here, R = any carbon backbone
Complete structural formula of a molecule represents all the atoms of molecule, types of bonds connecting atoms and how atoms are connected to each other.
Answer to Problem 11.8PP
Functional group in the given compound is '
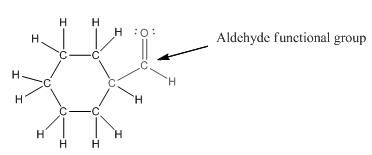
Explanation of Solution
The given compound is as follows:
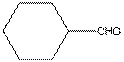
The given compound can also be drawn as:
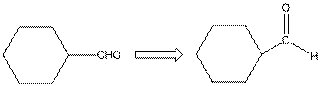
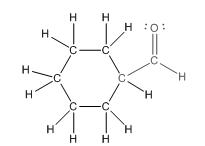
This compound contains a six membered ring and a
To draw the complete structure of the given skeletal structure, place carbon atom on each vertices and fulfill its valency with hydrogen atoms. Unlabelled vertex will represent carbon atom attached to number of hydrogen atoms required to fulfill its valency (i.e. four). Valence electron of oxygen is six. Out of six two electrons are used in making double bond with carbon atom. Then remaining four electrons will be available as lone pair.
So, complete structure of given compound is as follows:
(b)
Interpretation:
The functional group / groups should be identified and the complete structure with lone pairs on hetero-atoms of the following compound should be drawn:
Concept Introduction:
Organic molecules have some structural features in addition to
This table gives information about the compounds containing
| Type of Compound | General Structure |
| Aldehyde | 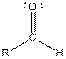 |
| Ketone | 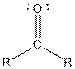 |
| Carboxylic acid | 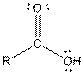 |
| Ester | 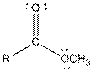 |
| Amide | 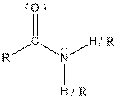 |
Here, R = any carbon backbone
Complete structural formula of a molecule represents all the atoms of molecule, types of bonds connecting atoms and how atoms are connected to each other.
Answer to Problem 11.8PP
Functional group in the given compound
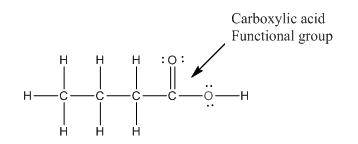
Explanation of Solution
The given compound is as follows:
The above compound can be drawn as:
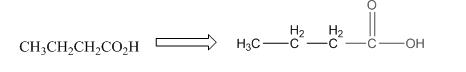
This compound contains four carbon and eight hydrogen atom with two oxygen atoms. The functional group is carboxylic acid containing an
To draw the complete structure of the given skeletal structure, place carbon atom on each vertices and fulfill its valency with hydrogen atoms. Unlabelled vertex will represent carbon atom attached to number of hydrogen atoms required to fulfill its valency (i.e. four). Valence electron of oxygen is six. Out of six two electrons of blue colored oxygen are used in making double bond with carbon atom. Then remaining four electrons will be available as lone pair.
Complete structure of the given compound is as follows:

(c)
Interpretation:
The functional group / groups should be identified and the complete structure with lone pairs on hetero-atoms of the following compound should be drawn:
Concept Introduction:
Organic molecules have some structural features in addition to
This table gives information about the compounds containing
| Type of Compound | General Structure |
| Aldehyde | 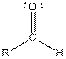 |
| Ketone | 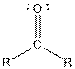 |
| Carboxylic acid | 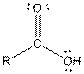 |
| Ester | 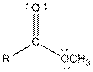 |
| Amide | 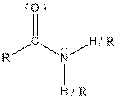 |
Here, R = any carbon backbone
Complete structural formula of a molecule represents all the atoms of molecule, types of bonds connecting atoms and how atoms are connected to each other.
Answer to Problem 11.8PP
Functional group in the given compound is 'ketone' and the complete structure of the compound is as follows:
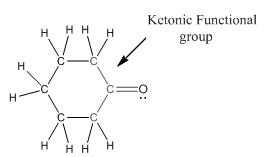
Explanation of Solution
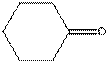
The given compound is as follows:
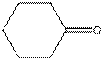
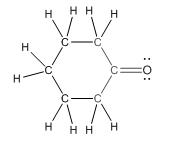
This compound contains six membered ring with one oxygen atom. The functional group is ketone containing a carbon-oxygen bond
To draw the complete structure of the given skeletal structure, place carbon atom on each vertices and fulfill its valency with hydrogen atoms. Unlabelled vertex will represent carbon atom attached to number of hydrogen atoms required to fulfill its valency (i.e. four). Valence electron of oxygen is six. Out of six two electrons of are used in making double bond with carbon atom. Then remaining four electrons will be available as lone pair.
Complete structure of the compound is as follows:
(d)
Interpretation:
The functional group / groups should be identified and the complete structure with lone pairs on hetero-atoms of the following compound should be drawn:
Concept Introduction:
Organic molecules have some structural features in addition to
This table gives information about the compounds containing
| Type of Compound | General Structure |
| Aldehyde | 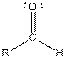 |
| Ketone | 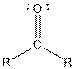 |
| Carboxylic acid | 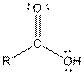 |
| Ester | 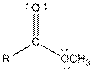 |
| Amide | 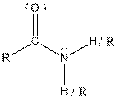 |
Here, R = any carbon backbone
Complete structural formula of a molecule represents all the atoms of molecule, types of bonds connecting atoms and how atoms are connected to each other.
Answer to Problem 11.8PP
Functional group in the given compound is 'ester' and the complete structure of the compound is as follows:
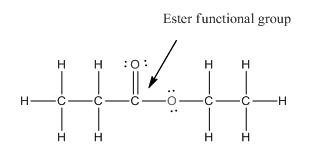
Explanation of Solution
The given compound is as follows:
This given compound can be drawn as follows:
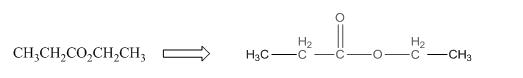
This compound contains an
To draw the complete structure of the given skeletal structure, place carbon atom on each vertices and fulfill its valency with hydrogen atoms. Unlabelled vertex will represent carbon atom attached to number of hydrogen atoms required to fulfill its valency (i.e. four).
Valence electron of oxygen is six. Out of six two electrons of blue colored oxygen are used in making double bond with carbon atom. Then remaining four electrons will be available as lone pair. For pink colored oxygen atom, one electron is used in making bond with carbonyl carbon atom and another electron is used in making bond with carbon backbone. Then remaining four will be present as lone pair.
The complete structure of the given compound is as follows:
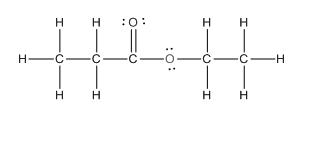
(e)
Interpretation:
The functional group / groups should be identified and the complete structure with lone pairs on hetero-atoms of the following compound should be drawn:
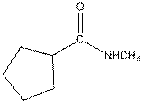
Concept Introduction:
Organic molecules have some structural features in addition to
This table gives information about the compounds containing
| Type of Compound | General Structure |
| Aldehyde | 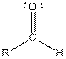 |
| Ketone | 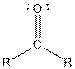 |
| Carboxylic acid | 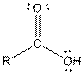 |
| Ester | 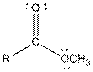 |
| Amide | 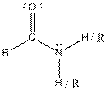 |
Here, R = any carbon backbone
Complete structural formula of a molecule represents all the atoms of molecule, types of bonds connecting atoms and how atoms are connected to each other.
Answer to Problem 11.8PP
Functional group in the given compound is an 'amide' and the complete structure of the compound is as follows:
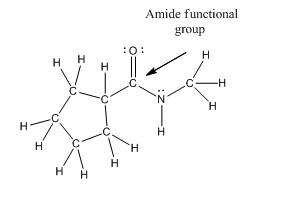
Explanation of Solution
The given compound is as follows:
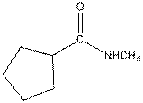
This compound contains an
To draw the complete structure of the given skeletal structure, place carbon atom on each vertices and fulfill its valency with hydrogen atoms. Unlabelled vertex will represent carbon atom attached to number of hydrogen atoms required to fulfill its valency (i.e. four).
Valence electron of oxygen is six. Out of six two electrons of oxygen atom are used in making double bond with carbon atom. Then remaining four electrons will be available as lone pair. Valence electron of nitrogen is five. Out of five, one electron is used in making bond with the carbonyl carbon atom, one electron is used in making bond with
The complete structure of the given compound is as follows:
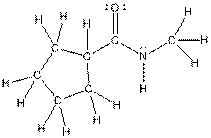
Want to see more full solutions like this?
Chapter 11 Solutions
General, Organic, and Biological Chemistry - 4th edition
- Nonearrow_forward4. Draw and label all possible isomers for [M(py)3(DMSO)2(CI)] (py = pyridine, DMSO dimethylsulfoxide).arrow_forwardThe emission data in cps displayed in Table 1 is reported to two decimal places by the chemist. However, the instrument output is shown in Table 2. Table 2. Iron emission from ICP-AES Sample Blank Standard Emission, cps 579.503252562 9308340.13122 Unknown Sample 343.232365741 Did the chemist make the correct choice in how they choose to display the data up in Table 1? Choose the best explanation from the choices below. No. Since the instrument calculates 12 digits for all values, they should all be kept and not truncated. Doing so would eliminate significant information. No. Since the instrument calculates 5 decimal places for the standard, all of the values should be limited to the same number. The other decimal places are not significant for the blank and unknown sample. Yes. The way Saman made the standards was limited by the 250-mL volumetric flask. This glassware can report values to 2 decimal places, and this establishes our number of significant figures. Yes. Instrumental data…arrow_forward
- 7. Draw a curved arrow mechanism for the following reaction. HO cat. HCI OH in dioxane with 4A molecular sievesarrow_forwardTry: Convert the given 3D perspective structure to Newman projection about C2 - C3 bond (C2 carbon in the front). Also, show Newman projection of other possible staggered conformers and circle the most stable conformation. Use the template shown. F H3C Br Harrow_forwardNonearrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





